Pharma QC
Aitia and UCB Announce Strategic Drug Discovery Collaboration in Huntington's Disease
Huntington's disease is a debilitating genetic disorder that affects the brain, causing gradual degeneration of nerve cells in specific areas of the brain,...
March 20, 2023 | News
Revolutionizing Pharmaceutical Quality Control: The Implementation of Digital Technologies
The pharmaceutical industry is constantly evolving, and new technologies are being introduced to streamline processes and improve quality contr...
March 17, 2023 | News
OGT first to achieve IVDR-certification for FISH probes
OGT, A Sysmex Group Company, announces that IVDR-certification has been granted for eight CytoCell® fluorescence in situ ...
March 13, 2023 | News
Improving Drug Safety and Regulatory Compliance: How AI is Revolutionizing Quality Control in Pharma Manufacturing
Quality control is an essential aspect of pharmaceutical manufacturing that ensures drugs are safe, effective, and meet regulatory standards. However, trad...
March 10, 2023 | Opinion
NIPPON EXPRESS HOLDINGS Strengthens Pharmaceutical Logistics Quality System Globally
NIPPON EXPRESS HOLDINGS, INC., based in Tokyo, has compiled the NX-Pharma Global Quality Manual, applicable as of Sunday, January 1, 2023, to uni...
March 09, 2023 | News
Estrella Biopharma's ARTEMIS® T Cell Therapy EB103 IND Cleared by FDA for B-Cell Lymphomas Clinical Trial
Estrella Biopharma, Inc. ("Estrella"), a biopharmaceutical company whose mission is to harness the evolutionary power of the human immune system to transfo...
March 09, 2023 | News
Catalent Appoints Sridhar Krishnan to Lead New Global Operational Excellence Strategy, “The Catalent Way”
Catalent, Inc. (NYSE: CTLT), the leader in enabling the development and supply of better treatments for patients worldwide, today announced the appointment...
March 06, 2023 | News
ExoCoBio Obtains GMP Licenses for Exosome Biopharmaceuticals and HA Dermal Fillers
ExoGMP™ is the world's largest exosome manufacturing facility in Osong, South Korea. Cellosome™ HA fillers are expected to be approved ...
March 02, 2023 | News
QMS in Pharma - Your Guide to Connecting Pharma Quality Data
Your quality team collects tons of data. But if you're like most pharma companies, that data is siloed in disparate systems or filing cab...
March 02, 2023 | News
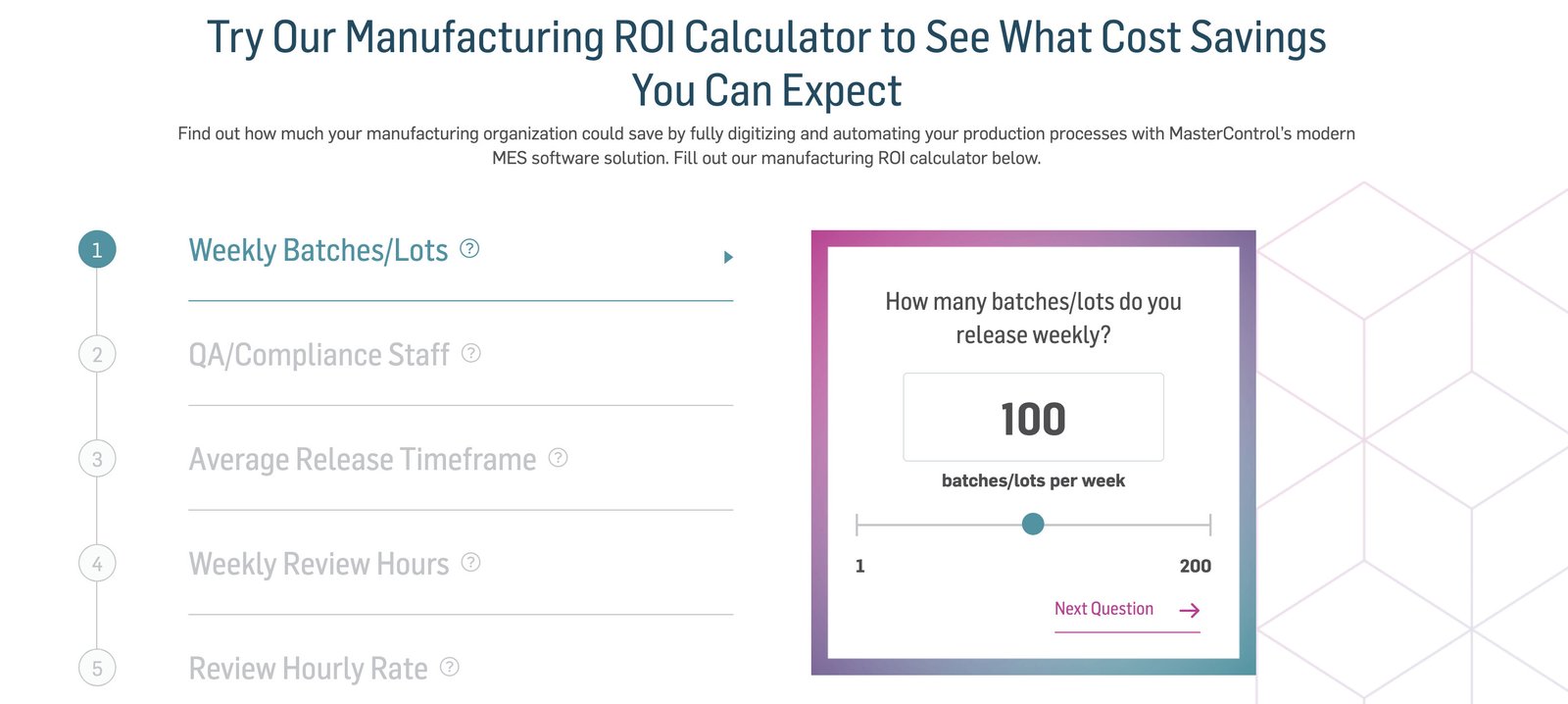
Manufacturing ROI Calculator - How Much Can Manufacturing Excellence Save Your Organisation?
Most manufacturers know that paper or disconnected systems on the factory floor make manufacturing operations slower and more prone to error, but the pai...
March 02, 2023 | News
AtomVie Global Radiopharma builds industry-leading CDMO facility in Ontario.
Currently located on the McMaster University campus, AtomVie successfully operates a global leading Contract Development and Manufacturing Organi...
March 02, 2023 | News
Körber and TetraScience partner to speed up productivity and increase data integrity for process scale-up and manufacturing operations
"Körber's PAS-X Savvy provides powerful analysis and modeling capabilities for the full biopharmaceutical production life cycle," said Simon Meffan-Ma...
March 02, 2023 | News
Eppendorf Centrifuge 5910 Ri is the first centrifuge in the market to receive My Green Lab ACT label.
By emphasizing Accountability, Consistency, and Transparency (ACT) around manufacturing, energy and water use, packaging, and end-of-life, the ACT label ma...
March 01, 2023 | News
Clean Cells launches activities at new biopharmaceutical quality control facility - largest in Europe
Company anticipates strong growth in demand and activity, focusing on new treatments such as cell and gene therapies, and offering new and improved service...
March 01, 2023 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News