Medtech Medical Devices
Copan Secures FDA 510(k) Clearance For PhenoMATRIX AI Driven Culture Plate Assessment Software
Copan Group announced that PhenoMATRIX®, its automated image assessment software used with WASPLab® full laboratory automation, rece...
February 19, 2026 | News
Neurophet Secures Third FDA Clearance For AI Driven Alzheimer’s Imaging Platform
Following AQUA and SCALE PET, the latest FDA clearance reinforces Neurophet's leadership in AI-based Alzheimer's disease imaging analysis Advanced ...
February 09, 2026 | News
Innovations In Magnetic Resonance Imaging Introduced By United Imaging
Tomorrow's technology. Artificial intelligence and the uCS platform The key to the innovations introduced by United Imaging is the combination of advanced...
February 05, 2026 | News
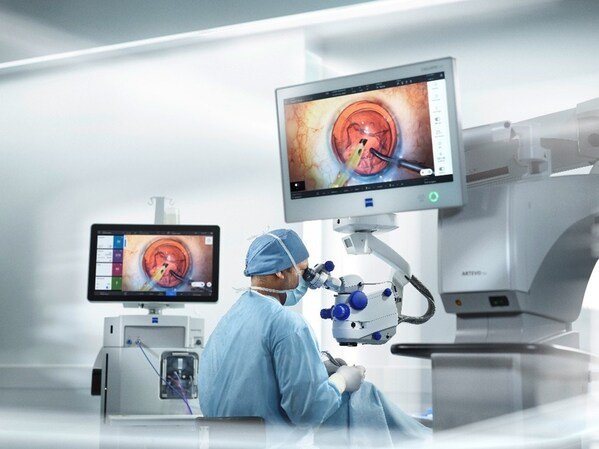
ZEISS Secures NMPA Approval For ARTEVO 750 And ARTEVO 850 Ophthalmic Surgical Microscopes In China
ZEISS ARTEVO 750 and ZEISS ARTEVO 850 surgical microscopes provide ophthalmic surgeons in China with end-to-end workflow integration to improve efficiency,...
February 04, 2026 | News
Imagine Devices Inc. Partners With Neotech Products To Advance Trinity Tube For Neonatal Critical Care
Imagine Devices Inc., a medical device company focused on advancing neonatal and pediatric critical care, announced a strategic partnership with Neotech Pr...
January 27, 2026 | News
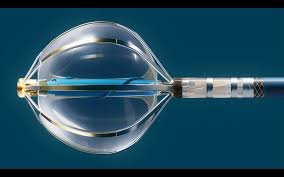
Ventaris Surgical Raises $30 Million Series A To Transform Kidney Stone Surgery
Ventaris Surgical, Inc., a San Carlos, CA-based medical device company developing a next-generation system for kidney stone treatment, announced the ...
January 12, 2026 | News
KORU Medical Submits FDA 510k For FreedomEDGE Use With PHESGO In HER2 Positive Breast Cancer
KORU Medical Systems, Inc. (NASDAQ: KRMD) (“KORU Medical” or the “Company”), a leading medical technology company focused on t...
January 01, 2026 | News
KLMBio Accelerates Global Expansion With High Value Human Tissue Based Bone Graft Portfolio
KLMBio, a manufacturer and exporter of human tissue-based medical devices, is accelerating its global business expansion by strengthening its high-value ti...
January 01, 2026 | News
StimLabs Secures FDA 510k Clearance For Theracor Advancing Wound Care Innovation
StimLabs announces that the U.S. Food & Drug Administration (FDA) has granted 510(k) clearance for Theracor, the first human umbilical cord-derived med...
December 31, 2025 | News
Abbott Secures FDA Approval For Volt PFA System, Advancing Atrial Fibrillation Treatment In The United States
Abbott's Volt™ PFA System, the latest generation of cardiac ablation technology, is designed for people battling heart rhythm d...
December 29, 2025 | News
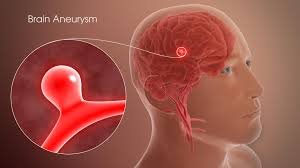
Galaxy Therapeutics Completes Enrollment In Landmark U.S. IDE Trial For Brain Aneurysm Device
Galaxy Therapeutics, a privately held, clinical-stage medical device company focused on treating brain aneurysms, announced it has successfully completed e...
December 26, 2025 | News
UK Hospitals Adopt AI Tool MEMORI To Strengthen Early Detection Of Hospital Acquired Infections
Sanome, the health tech company behind MEMORI, an AI-powered clinical decision support tool that helps clinicians detect hospital-acquired infections (HAIs...
December 18, 2025 | News
CooperVision Opens Regional Service Centre in Singapore to Strengthen APAC Supply and Access
Driving faster delivery and broader portfolio access across the region. CooperVision Asia Pacific, in collaboration with CEVA Logistics, has opened a new ...
December 02, 2025 | News
Genesis MedTech And NUHS Launch AI Innovation Partnership To Advance The Future Of Surgery
Genesis MedTech Group and the National University Health System (NUHS) have formed a strategic partnership to advance artificial intelligence (AI...
November 19, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News