Countries South Korea
Catalent And S Biomedics Partner To Advance Stem Cell Therapy For Parkinsons Disease
Catalent and S.Biomedics announced a strategic partnership to support the development and manufacturing of TED‑A9, S.Biomedics’ allogeneic pluripot...
February 23, 2026 | News
Jyong Biotech Strengthens Preventive Medicine Position With Positive Phase II Data And Asian Expansion
Comprehensive review of recent milestones highlights statistically significant efficacy in prostate cancer prevention, discovery of novel lipid-modul...
February 16, 2026 | News
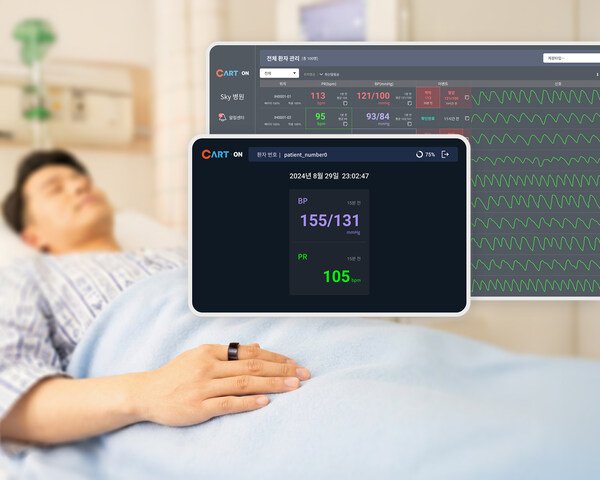
Sky Labs Launches CART ON Ring Based Ward Blood Pressure Monitoring System
Establishment of a blood pressure monitoring lineup that addresses every environment, from daily life to hospital wards Sky Labs (CEO Jack Byunghwan Lee) ...
February 12, 2026 | News
GCCL And Medicover Integrated Clinical Services Sign MOU To Strengthen Multinational Trial Delivery
GCCL Co., Ltd. (GCCL), a data-driven clinical trial services provider, and Medicover Integrated Clinical Services (MICS), part of Medicover, an internation...
February 12, 2026 | News
Samsung Biologics PCF System Validated Against Global Carbon Accounting Standards
Samsung Biologics' PCF system validated in line with globally established ISO and PAS standards Validation reflects continued efforts to establish ...
February 09, 2026 | News
Korean Courts Uphold Kolmar Korea Victory In Sunscreen Technology Misappropriation Case
- Korean courts order Intercos Korea to bear full legal costs, affirming both civil and criminal liability Kolmar Korea has secured a decisive legal ...
February 09, 2026 | News
Neurophet Secures Third FDA Clearance For AI Driven Alzheimer’s Imaging Platform
Following AQUA and SCALE PET, the latest FDA clearance reinforces Neurophet's leadership in AI-based Alzheimer's disease imaging analysis Advanced ...
February 09, 2026 | News
Samsung Biologics Partners With Coalition for Epidemic Preparedness Innovations To Boost Outbreak Ready Vaccine Manufacturing
The Coalition for Epidemic Preparedness Innovations (CEPI) and Samsung Biologics (KRX: 207940.KS), a leading contract development and manufacturing organiz...
February 05, 2026 | News
SK bioscience Advances Early Clinical RSV Preventive Monoclonal Antibody Licensed From Gates Medical Research Institute
SK to advance novel early-clinical stage RSV preventive monoclonal antibody in-licensed from Gates Medical Research Institute Exclusive rights to commer...
February 05, 2026 | News
Samsung Biologics Earns EcoVadis Platinum Sustainability Rating
Samsung Biologics (KRX: 207940.KS), a leading contract development and manufacturing organization (CDMO), announced that it has received the EcoVadis...
January 30, 2026 | News
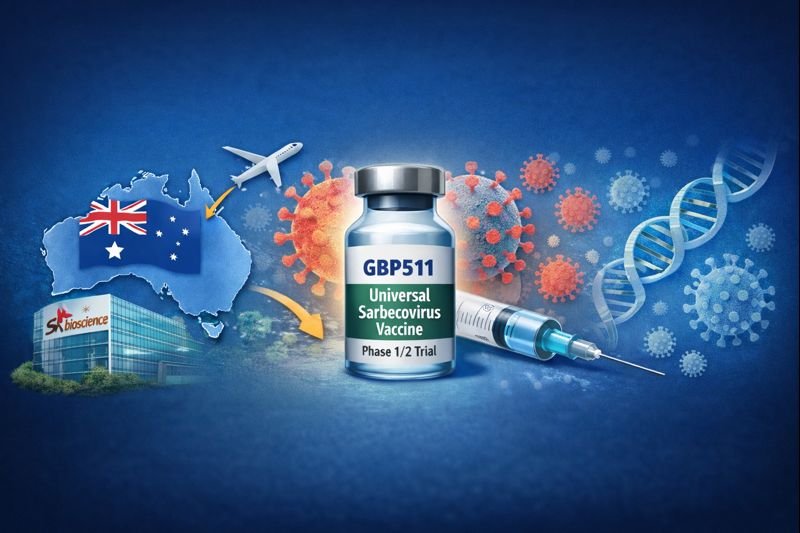
SK Bioscience Launches Global Phase1/2 Trial In Australia For Universal Sarbecovirus Vaccine GBP511
SK bioscience announced the initiation of a global Phase 1/2 clinical trial in Australia for GBP511, a vaccine candidate targeting the sarbecovirus subgenu...
January 30, 2026 | News
Hanmi Pharmaceutical Advances Global GLP 1 Strategy With Mexico Distribution Agreement For Efpeglenatide
Hanmi is accelerating its global market entry with efpeglenatide, Korea's first domestically developed GLP-1 class obesity and metabolic disease ...
January 29, 2026 | News
SK Bioscience Advances Zaire Ebolavirus Vaccine Development With CEPI Backed Global Collaboration
SK bioscience announced that it is advancing the development of a Zaire ebolavirus vaccine under a new collaboration supported by the Coalition for Epidemi...
January 23, 2026 | News
Samsung Biologics Strengthens Long Term Growth Platform With Solid 2025 Financial Performance
Recorded Q4'25 revenue of KRW 1,286 billion and FY'25 revenue of KRW 4,557 billion Maintained momentum through consistent ...
January 22, 2026 | Company results
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News