BioPharma Drug Approval
Ractigen Therapeutics Receives FDA Orphan Drug Designation for RAG-21, a Novel siRNA Therapy for FUS-ALS
Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing innovative therapies, announced that the U.S. Food and Drug Administ...
November 20, 2024 | News
Epigenic Therapeutics Secures Approval in New Zealand for World’s First Epigenetic Therapy Clinical Trial for Chronic Hepatitis B
Epigenic Therapeutics, a leading biotechnology company dedicated to developing next-generation gene modulation therapy, announced that it has received appr...
November 20, 2024 | News
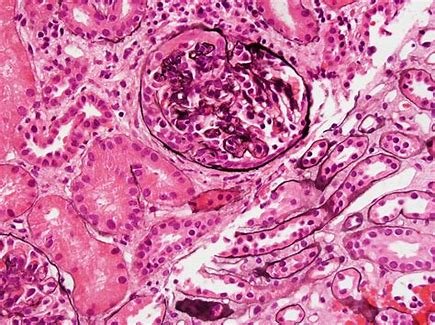
Everest Medicines' NEFECON® Gains Full Approval in South Korea for IgA Nephropathy Treatment
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing an...
November 19, 2024 | News
Acepodia Secures FDA IND Clearance for ACE1831, Pioneering Gamma Delta T Cell Therapy in Autoimmune Disease
IND clearance paves the way for Acepodia to enter the clinic for the first time with its autoimmune program ACE1831 is an allogeneic gamma delta T...
November 18, 2024 | News
Ascentage Pharma’s Lisaftoclax NDA Accepted for Priority Review in China: A Potential First for Bcl-2 Inhibitors
Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unm...
November 18, 2024 | News
Mabwell's Nectin-4 Targeting ADC Secures IND Approval for Clinical Trials in Perioperative Urothelial Carcinoma and Advanced Solid Tumors
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its novel Nectin-4 targeting ADC (R&D code: 9MW...
November 15, 2024 | News
Taiwan's Caliway Biopharmaceuticals Receives EMA Orphan Drug Designation for CBL-514 in Dercum’s Disease Treatment
CBL-514 is the first and only drug to receive EMA Orphan Drug Designation for Dercum's disease treatment.- CBL-514 received both FDA Orphan Drug...
November 14, 2024 | News
Alphamab Oncology’s JSKN033 Receives Fast-Track Approval from Shanghai Drug Authority for Innovative Cancer Therapy Clinical Trials
Alphamab Oncology (stock code: 9966.HK) announced that a clinical trial (Study ID: JSKN033-102) of JSKN033, a high-concentration subcutaneous co-formulatio...
November 13, 2024 | News
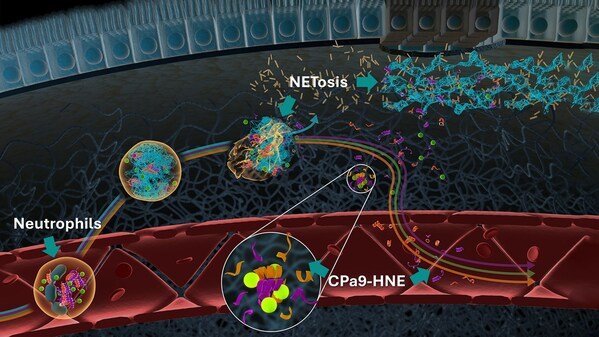
Nordic Bioscience Secures FDA Letter of Support for CPa9-HNE Biomarker in Advancing IBD Clinical Trials
Nordic Bioscience, a leading biomarker company, announced that its CPa9-HNE biomarker assay received a Letter of Support (LoS) from the U.S. Food and Drug ...
November 12, 2024 | News
Australia’s Neurizon Therapeutics Secures Positive EMA Opinion for Orphan Medicinal Product Designation of NUZ-001 in ALS
Notice of positive opinion received from the European Medicines Agency for Orphan Medicinal Product Designation for NUZ-001 in Amyotrophic Lateral S...
November 12, 2024 | News
Harbour BioMed Files IND Application in China for Novel Antibody Therapy HBM9378/SKB378 Targeting COPD
Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel ant...
November 11, 2024 | News
Dizal Submits NDA to U.S. FDA for Sunvozertinib as a New Treatment Option for Advanced NSCLC with EGFR Exon 20 Insertion Mutations
Dizal (SSE:688192), a biopharmaceutical company committed to developing novel medicines for the treatment of cancer and immunological diseases, announ...
November 11, 2024 | News
China’s Leads Biolabs Secures FDA Orphan Drug Designation for LBL-034 in Multiple Myeloma Treatment
Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-034, a humanized bispecific T-cell engaging antibody target...
November 07, 2024 | News
DX&VX Selected for South Korea's ARPA-H Project to Pioneer Room-Temperature mRNA Vaccine Storage
Pioneering Korea's ARPA-H Project: Focused on mRNA Vaccine Room-Temperature Storage and Extra-Long-Term Preservation Creating a Platform for Prolonged R...
November 04, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News