BioPharma Drug Approval
Everest Medicines' NEFECON® sNDA Accepted by China’s NMPA, Paving the Way for First Full Approval of IgA Nephropathy Treatment
Everest Medicines, a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeuti...
July 18, 2024 | News
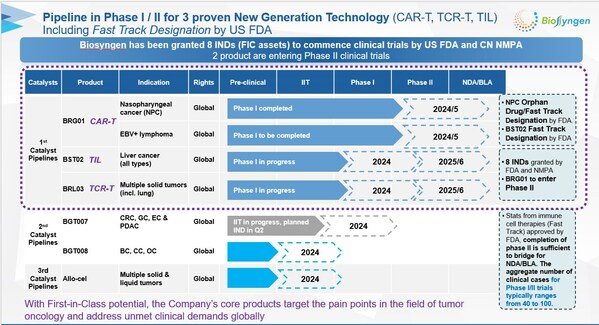
Biosyngen's Groundbreaking CAR-T Therapy BRG01 Approved for Phase II Clinical Trial in China
Biosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation ...
July 17, 2024 | News
Mabwell's Novel Nectin-4 ADC Approved for Phase II Clinical Trial in Treating Triple-Negative Breast Cancer
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821)...
July 16, 2024 | News
ADM Korea to Submit IND for Niclosamide-Based Combination Therapy for Prostate Cancer
IND for clinical study for combination therapy with hormone therapy in prostate cancer patients to be submitted in August, 2024. Niclosamide-based metab...
July 12, 2024 | News
Eisai and Biogen Announce Approval of LEQEMBI® (Lecanemab) for Alzheimer’s Disease Treatment in Hong Kong
LEQEMBI is now available for patients with mild cognitive impairment (MCI) or mild dementia, collectively referred to as early AD, the population in which ...
July 11, 2024 | News
XPOVIO® Secures NMPA Approval for Treatment of R/R DLBCL in China, Expanding Its Indications
- Following its initial approval for the treatment of relapsed/refractory multiple myeloma (R/R MM), XPOVIO® has now received approval as a monoth...
July 08, 2024 | News
Eleva's Factor H Receives Orphan Drug Designation from European Commission for Treating C3 Glomerulopathy
Eleva, a pioneer in unlocking difficult-to-produce biologics based on a breakthrough manufacturing platform, announced today that the European Commis...
July 04, 2024 | News
European Commission Approves OBGEMSA™ (Vibegron) for Overactive Bladder Treatment by Pierre Fabre Laboratories
The European Commission (EC) has authorized the marketing of OBGEMSA™ (vibegron) by Pierre Fabre Laboratories for the symptomatic treatment of overac...
July 01, 2024 | News
South Korea Approves Reimbursement for Antengene's XPOVIO®, Expanding Access to Relapsed/Refractory Multiple Myeloma Treatment
- XPOVIO® is the first XPO1 inhibitor approved for reimbursement by South Korea's National Health Insurance Service (NHIS) for the treat...
June 27, 2024 | News
Simcere Zaiming's Enlituo® Receives NMPA Approval for mCRC Treatment
Simcere Zaiming, an innovative oncology company and a subsidiary of Simcere Pharmaceutical Group (2096.HK), announced that Enlituo® (generic name: cetu...
June 27, 2024 | News
EU Approves HUTCHMED's FRUZAQLA® for Previously Treated Metastatic Colorectal Cancer
— Approval for previously treated metastatic colorectal cancer based on results from positive, global, Phase III FRESCO-2 Trial — — FRUZ...
June 24, 2024 | News
Sarepta Therapeutics Secures FDA Approval for Expanded Use of ELEVIDYS in Duchenne Muscular Dystrophy Patients
Sarepta Therapeutics, the leader in precision genetic medicine for rare diseases, today announced U.S. Food and Drug Administration (FDA) approv...
June 21, 2024 | News
Dizal's Golidocitinib Approved in China as First JAK1-Only Inhibitor for Relapsed or Refractory Peripheral T-Cell Lymphoma
Golidocitinib is a first-in-class Janus kinase 1 (JAK1) only inhibitor approved for the treatment of r/r PTCL based on results from the multinational piv...
June 20, 2024 | News
Menarini Expands Partnership with Pharmacosmos to Bring Innovative Iron Deficiency Treatment to Singapore and Malaysia
Menarini Asia-Pacific (Menarini) announced that it has expanded its partnership with Pharmacosmos A/S (Pharmacosmos), to include exclusive rights to ...
June 19, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News