BioPharma Clinical Trials
Endeavor BioMedicines’ Taladegib Becomes First Investigational IPF Therapy to Receive EMA PRIME Designation
Taladegib is the first and only investigational IPF therapy to receive PRIME designation Phase 2b WHISTLE-PF trial is on track to complete enrollment ...
November 04, 2025 | News
NovaBridge Subsidiary Visara Assigns Exclusive License for VIS-101 to Everest Medicines to Advance Treatment for Wet AMD Across Asia
NovaBridge subsidiary Visara assigned its exclusive license to Everest Medicines to accelerate the development of potential best-in-class therapy for wet...
November 03, 2025 | News
Alpha Fusion Initiates Phase I Trial of Alpha-Emitting Radiopharmaceutical af-001 for Differentiated Thyroid Cancer
– Aiming to establish af-001 as a new therapeutic option for radioactive iodine (RAI)-naïve patients with differentiated thyroid cancer – ...
November 03, 2025 | News
Shanghai Zhimeng Biopharma Begins Phase 2/3 Trial of Next-Generation Potassium Channel Opener for ALS
Previously (in July 2025), CB03-154 received approval from the China Center for Drug Evaluation (CDE) to conduct the ALS phase 2/3 adaptive clin...
November 03, 2025 | News
Suvoda Launches Unified Patient App to Simplify Clinical Trial Participation and Boost Retention
Suvoda (recently merged under common ownership with Greenphire), a global clinical trial technology company specializing in software solutions t...
October 30, 2025 | News
Thermo Fisher to Acquire Clario for US$8.9 Billion to Expand Digital and AI-Driven Clinical Trial Capabilities
Expected to be immediately accretive to Adjusted Earnings Per Share (EPS)¹ after close Attractive return profile given high growth and strong margin ...
October 30, 2025 | News
Myrio Therapeutics Partners with University of Pennsylvania and NYU Langone to Advance Next-Generation T Cell Immunotherapies
Myrio Therapeutics, the University of Pennsylvania Perelman School of Medicine, and NYU Langone Health have entered a research collaboration to...
October 29, 2025 | News
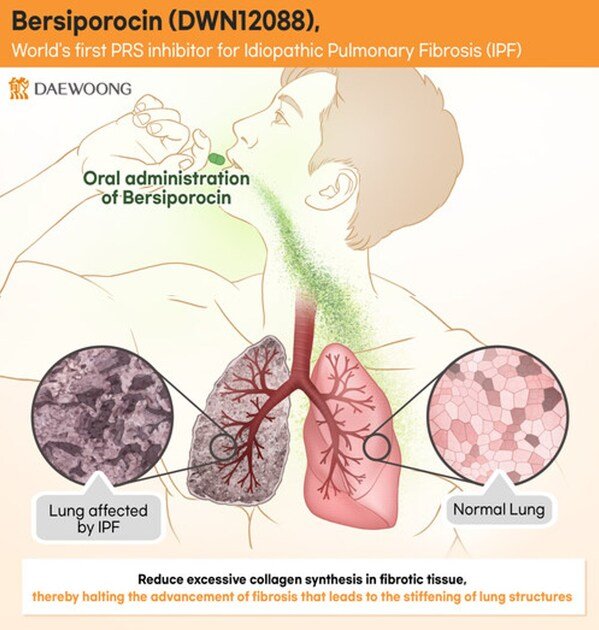
Daewoong Pharmaceutical’s Bersiporocin Clears Third IDMC Safety Review, Global Phase 2 IPF Trial to Continue
Third Independent Data Monitoring Committee (IDMC) review confirms safety and continuation of the study 94 patients enrolled out of 102 planned across K...
October 28, 2025 | News
OncoC4 and BioNTech’s Gotistobart Receives Breakthrough Therapy Designation in China for Squamous Non-Small Cell Lung Cancer
Designation granted for gotistobart (BNT316/ONC392) for the treatment of patients with squamous non-small cell lung cancer (sqNSCLC), an aggressive subtype...
October 28, 2025 | News
Alphamab Oncology Doses First Patient in Global First PD-L1/αvβ6 Bispecific ADC Clinical Trial
Alphamab Oncology (stock code: 9966.HK) announced that the first patient has been successfully dosed at Sun Yat-sen University Cancer Center in the phase I...
October 27, 2025 | News
EMA Grants Orphan Drug Designation to Ribo’s RBD1016 for Treatment of Hepatitis D Virus Infection
Suzhou Ribo Life Science Co., Ltd. and Ribocure Pharmaceuticals AB (Ribo), announced that the European Medicines Agency (EMA) has granted O...
October 27, 2025 | News
Venus Medtech Completes Patient Enrollment in Cardiovalve TARGET Study for Tricuspid Valve Replacement
Venus Medtech (Hangzhou) Inc. (2500.HK, hereinafter referred to as the "Company") announced the successful completion of patient enrollmen...
October 27, 2025 | News
METiS TechBio’s AI-Designed MTS-004 Meets Phase III Success in China, Targeting Pseudobulbar Affect
MTS-004 Poised to Fill an Unmet Need in the Treatment of Pseudobulbar Affect (PBA) METiS TechBio, a global leader in AI-driven nanodelivery and formulatio...
October 24, 2025 | News
Takeda and Innovent Biologics Announce Global Collaboration for Two Late-Stage Oncology Programs Valued at Over US$1.2 Billion
Takeda to Receive Rights to Two Next-Generation Late-Stage Investigational Medicines, Worldwide Outside of Greater China, and an Exclusive Option to Li...
October 23, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News