Drug Discovery
Alphamab Oncology’s JSKN033 Receives Fast-Track Approval from Shanghai Drug Authority for Innovative Cancer Therapy Clinical Trials
Alphamab Oncology (stock code: 9966.HK) announced that a clinical trial (Study ID: JSKN033-102) of JSKN033, a high-concentration subcutaneous co-formulatio...
November 13, 2024 | News
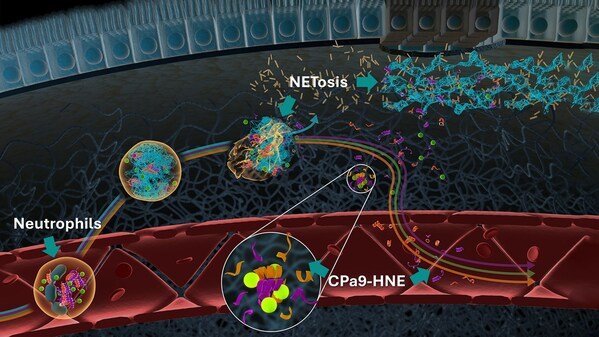
Nordic Bioscience Secures FDA Letter of Support for CPa9-HNE Biomarker in Advancing IBD Clinical Trials
Nordic Bioscience, a leading biomarker company, announced that its CPa9-HNE biomarker assay received a Letter of Support (LoS) from the U.S. Food and Drug ...
November 12, 2024 | News
Australia’s Neurizon Therapeutics Secures Positive EMA Opinion for Orphan Medicinal Product Designation of NUZ-001 in ALS
Notice of positive opinion received from the European Medicines Agency for Orphan Medicinal Product Designation for NUZ-001 in Amyotrophic Lateral S...
November 12, 2024 | News
Harbour BioMed Files IND Application in China for Novel Antibody Therapy HBM9378/SKB378 Targeting COPD
Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel ant...
November 11, 2024 | News
Dizal Submits NDA to U.S. FDA for Sunvozertinib as a New Treatment Option for Advanced NSCLC with EGFR Exon 20 Insertion Mutations
Dizal (SSE:688192), a biopharmaceutical company committed to developing novel medicines for the treatment of cancer and immunological diseases, announ...
November 11, 2024 | News
BioIVT and KRISHGEN BioSystems Forge Exclusive Agreement to Expand Access to High-Quality Biospecimen Solutions Across South Asia
-BioIVT, a global research partner and biospecimen solutions provider for drug and diagnostic development, announced an exclusive agreement* with KRI...
November 07, 2024 | News
China’s Leads Biolabs Secures FDA Orphan Drug Designation for LBL-034 in Multiple Myeloma Treatment
Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-034, a humanized bispecific T-cell engaging antibody target...
November 07, 2024 | News
Medidata Introduces Oncology and Vaccine Solutions to Streamline Phase II and III Trials with FDA-Aligned Patient-Centric Design
The new products combine Medidata's diverse technologies and experience from thousands of previous trials to streamline the management of Phase II an...
November 05, 2024 | News
Breast Cancer Drug Shows Promise for Treating Hereditary Hemorrhagic Telangiectasia, a Rare Vascular Disorder
A drug commonly used to treat breast cancer might be able to help people living with hereditary hemorrhagic telangiectasia (HHT) – a rare genetic ble...
November 05, 2024 | News
Senhwa Biosciences Appoints Dr. Yiu-Lian Fong to Board of Directors for Strategic Growth in Oncology and Precision Medicine
Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious disea...
November 04, 2024 | News
Taiwan’s CStone Secures UK Approval for Sugemalimab as First-Line Treatment for Metastatic NSCLC
This approval marks the second international marketing authorization for sugemalimab outside of China, following its recent approval by the Eur...
November 01, 2024 | News
Taiwan’s Formosa Pharmaceuticals Partners with Portugal’s DÁVI Farmacêutica to Introduce APP13007 for Post-Surgical Ocular Pain Relief in Europe
Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TW) announced that the company has entered into an exclusive licensing agreement with DÁVI Far...
November 01, 2024 | News
Thermo Fisher’s $22M OSD Investment: Shervin Hebbi on Accelerating Drug Development with Cutting-Edge Technology
In an exclusive Q&A with BioPharma APAC, Shervin Hebbi, Vice President of Oral Solid Dose (OSD) – North America at Thermo Fisher ...
November 01, 2024 | Interview
Hong Kong’s Akeso Biopharma Enrolls First Patient in Pioneering Phase III Trial for Advanced Head and Neck Cancer Therapy
Akeso Biopharma (9926.HK) is pleased to announce the enrollment of the first patient in its randomized, controlled, multicenter Phase III clinical study (A...
October 31, 2024 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks