Drug Discovery
Akeso's Penpulimab and Anlotinib Combo Accepted by NMPA for First-Line Advanced Liver Cancer Treatment
Akeso, Inc. (9926.HK) ("Akeso" or the "Company") announced that the National Medical Products Administration (NMPA) of China has accep...
November 25, 2024 | News
Boehringer Ingelheim Introduces VETMEDIN® Solution: A Liquid Option for Managing Canine Heart Failure
Heart disease in dogs is almost as common as it is in humans, affecting approximately 10 percent of dogs in their lifetime Previous studies have shown t...
November 25, 2024 | News
NVIDIA’s BioNeMo Framework Unites Pharma, TechBio, and AI Leaders to Advance Drug Discovery
Argonne National Laboratory, Flagship Pioneering, Terray, Weights & Biases and Dozens More Organizations Among Contributors Advancing Biomolecular Scie...
November 22, 2024 | News
Lepu Biopharma and DP Technology Achieve Milestone in ADC Drug Development Through AI-Powered Collaboration
This partnership has effectively integrated DP's ADC Linker-Payload design platform with Lepu's ADC technology development platform, capitalizing on t...
November 22, 2024 | News
Converge Bio Secures $5.5M Seed Funding to Transform Drug Discovery with Generative AI
Converge Bio, a generative AI platform for accelerated drug discovery and development, announced it has raised $5.5 million in seed funding, ...
November 21, 2024 | News
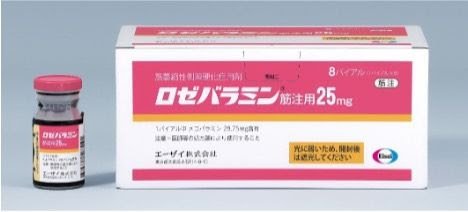
Eisai Launches Rozebalamin® in Japan, Offering New Hope for ALS Patients
Eisai Co., Ltd. announced that the amyotrophic lateral sclerosis (ALS) treatment “Rozebalamin® for Injection25 mg” (mecobalamin) has ...
November 21, 2024 | News
GBI Biomanufacturing Expands Services with Automated Sterile Fill-Finish Capabilities
GBI Biomanufacturing (GBI), one of the world's longest serving contract development and manufacturing organizations (CDMO), is pleased to announce the expa...
November 21, 2024 | News
Ractigen Therapeutics Receives FDA Orphan Drug Designation for RAG-21, a Novel siRNA Therapy for FUS-ALS
Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing innovative therapies, announced that the U.S. Food and Drug Administ...
November 20, 2024 | News
Everest Medicines' NEFECON® Gains Full Approval in South Korea for IgA Nephropathy Treatment
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing an...
November 19, 2024 | News
Acepodia Secures FDA IND Clearance for ACE1831, Pioneering Gamma Delta T Cell Therapy in Autoimmune Disease
IND clearance paves the way for Acepodia to enter the clinic for the first time with its autoimmune program ACE1831 is an allogeneic gamma delta T...
November 18, 2024 | News
Ascentage Pharma’s Lisaftoclax NDA Accepted for Priority Review in China: A Potential First for Bcl-2 Inhibitors
Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unm...
November 18, 2024 | News
Mabwell's Nectin-4 Targeting ADC Secures IND Approval for Clinical Trials in Perioperative Urothelial Carcinoma and Advanced Solid Tumors
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its novel Nectin-4 targeting ADC (R&D code: 9MW...
November 15, 2024 | News
SEKISUI Diagnostics Completes £15.7 Million cGMP Expansion for Enhanced Microbial Drug Substance Manufacturing in the UK
SEKISUI Diagnostics' microbial CDMO business announces it has completed construction of its £15.7 million ($20.7 million) cGMP capacity expansion&nbs...
November 15, 2024 | News
Taiwan's Caliway Biopharmaceuticals Receives EMA Orphan Drug Designation for CBL-514 in Dercum’s Disease Treatment
CBL-514 is the first and only drug to receive EMA Orphan Drug Designation for Dercum's disease treatment.- CBL-514 received both FDA Orphan Drug...
November 14, 2024 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks