BioPharma Clinical Trials
Innovent Biologics Receives FDA Fast Track Designation for PD-1/IL-2α Bispecific Antibody in Advanced Melanoma Treatment
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality me...
September 04, 2024 | News
Simcere Zaiming and TargetRx Forge Strategic Partnership for Groundbreaking ALK/ROS1 Dual Inhibitor in China
Simcere Zaiming, an innovative oncology company under Simcere Pharmaceutical Group (2096.HK), announced a collaboration agreement with Shenzhen TargetRx In...
September 03, 2024 | News
Nxera Pharma and Neurocrine Biosciences Report Positive Phase 2 Results for NBI-1117568 in Schizophrenia Treatment
Nxera Pharma – formerly known as Sosei Group or Sosei Heptares – notes the announcement by its partner Neurocrine Biosciences Inc. (&ldqu...
August 30, 2024 | News
Bayer Enrolls First Patient in Global Phase III SOHO-02 Trial Evaluating BAY 2927088 as First-Line Therapy for HER2-Mutant NSCLC
Bayer announced that the first patient has been enrolled in the global Phase III SOHO-02 trial, an open-label, randomized, multicenter clinical trial, asse...
August 30, 2024 | News
Merck Initiates Phase III MyClad Trial Dosing First Patient in Study of Oral Cladribine for Generalized Myasthenia Gravis
Merck, a leading science and technology company, announced the first patient has been dosed in the Phase III MyClad trial (NCT06463587) evaluating the effi...
August 30, 2024 | News
Yunovia Gains MFDS Clearance for Phase 1 MAD Study of Oral GLP-1 Agonist for Diabetes and Obesity
Yunovia, a drug R&D subsidiary of Ildong Pharmaceutical Group, announced on the 26th that the Ministry of Food and Drug Safety (MFDS) of Korea has clea...
August 28, 2024 | News
Shilpa Medicare Completes Phase 1 Trial for sRbumin®, India’s First Recombinant Human Albumin
Shilpa Medicare Limited (BSE: 530549) (NSE: SHILPAMED) proudly announces the successful completion of its Phase 1 clinical trial for its flagship prod...
August 28, 2024 | News
Telix Pharmaceuticals Submits NDA to FDA for Innovative Brain Cancer Imaging Agent Pixclara®
Telix Pharmaceuticals announces it has submitted a New Drug Application (NDA) to the United States (U.S.) Food and Drug Administration (FDA) for ...
August 28, 2024 | News
ArkBio CEO Presents Promising IPF Drug AK3280 at 8th Annual IPF Summit in Boston
Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio"), a privately held, clinical-stage biopharmaceutical company today announced that Dr. Jim Wu, Chief...
August 27, 2024 | News
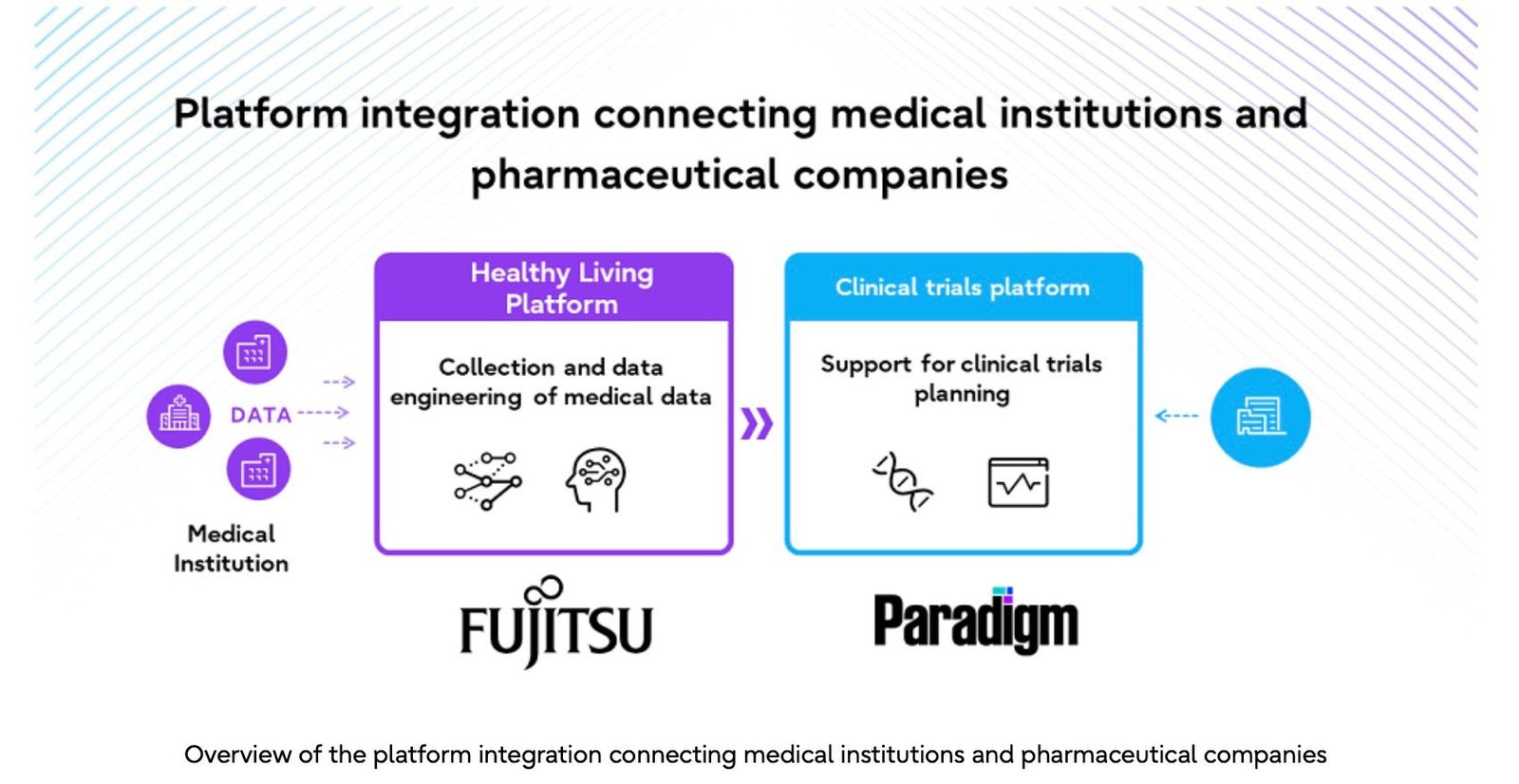
Fujitsu Launches Major Initiative to Attract Global Clinical Trials to Japan, Tackling the Nation's 'Drug Loss' Issue
Fujitsu announced that it will begin initiatives to attract global clinical trials to Japan and tackle the ‘drug loss’ issue by working w...
August 26, 2024 | News
Mabwell Receives NMPA Approval to Initiate Phase III Study of 9MW2821 in Recurrent or Metastatic Cervical Cancer
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its submission to the Center for Drug Evaluation (C...
August 26, 2024 | News
CanariaBio Completes Enrollment for Phase 2 Study of Oregovomab with Chemotherapy in Advanced Ovarian Cancer
CanariaBio Inc, a leading late-stage biotechnology company, announces the successful completion of enrollment of 88 patients in a randomized Phase 2 study ...
August 26, 2024 | News
AiViva Biopharma Completes Enrollment in Phase 1 Trial of AIV007 for Wet AMD and DME
AiViva Biopharma Inc., a clinical-stage biotechnology company, announced that it has fully enrolled and completed dosing the last patient in a Phase 1 tria...
August 26, 2024 | News
Everest Medicines Launches First Clinical Trial for Personalized mRNA Cancer Vaccine EVM16 in China
Everest Medicines , a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeut...
August 22, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News