Medtech Medical Devices
Thermo Fisher Launches Next-Gen TruNarc™ Analyzers to Strengthen Global Fight Against Illicit Drugs
Thermo Fisher Scientific, the world leader in serving science, introduced the Thermo Scientific™ TruNarc™ Delta and Tau Handheld Na...
May 23, 2025 | News
Oliver Healthcare Packaging Expands Asia-Pacific Footprint with New Manufacturing Facility in Johor, Malaysia
Oliver Healthcare Packaging (“Oliver”), a leading healthcare company driving quality and innovation in medical packaging, has officially opened...
May 15, 2025 | News
iotaMotion Marks International Milestone with First Use of iotaSOFT® System in Zurich Cochlear Implant Study
iotaMotion, Inc., a leader in robotic-assisted systems for cochlear implant surgery, announced the first use of the iotaSOFT® Insertion Syst...
May 14, 2025 | News
Nordic Bioscience and Roche Launch Elecsys PRO-C3 Test to Advance Liver Fibrosis Assessment on cobas Analyzers
Nordic Bioscience announces that its PRO-C3 test is launched by Roche Diagnostics on their cobas analysers. The Roche Elecsys® PRO-C3, used w...
May 09, 2025 | News
Transverse Medical Completes First Patient Series in Feasibility Study of POINT-GUARD™ CEP Device in Australia
Transverse Medical Inc. is excited to announce the completion of the first series of patients under its Feasibility Clinical Study at the prestigious Victo...
April 22, 2025 | News
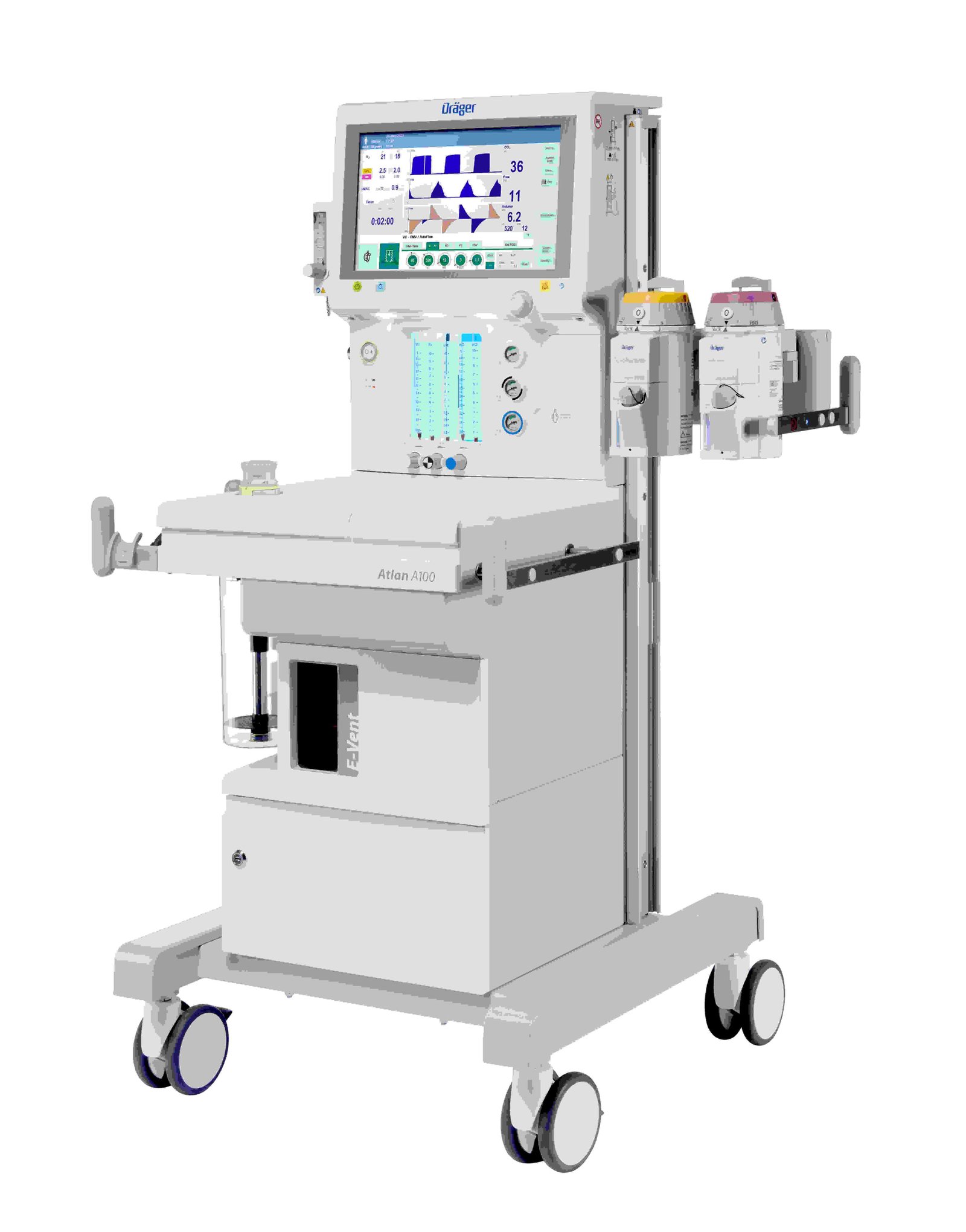
Dräger Launches Atlan® A100 Anesthesia Workstation in India to Elevate Perioperative Care
Dräger, a global leader in medical and safety technology, announces the launch of the Atlan® A100 anesthesia workstation in India, an innovative a...
April 10, 2025 | News
Amcor Launches Malaysia’s First Advanced Healthcare Packaging Facility with Cutting-Edge Coating Technology
New facility enhances supply chain security, reduces lead times and supports customers' growth First in Asia equipped with advanced air knife co...
April 10, 2025 | News
Logistics4Pharma Expands Customs Services to Strengthen Global Pharma Supply Chains
Logistics4Pharma, a Frankfurt-based GDP-certified pharma logistics provider, has expanded its service portfolio. In addition to Temporary Storage und...
April 09, 2025 | News
Nelipak® Strengthens Direct Presence and Capabilities in Asia-Pacific to Support Growing Demand for Healthcare Packaging
– Nelipak® Corporation ("Nelipak®"), a leading global manufacturer of packaging solutions for medical device, diagnostic, phar...
April 07, 2025 | News
Peijia Medical and dsm-firmenich Forge Strategic Innovation Partnership to Advance Next-Gen Polymer Heart Valve Technologies
Peijia Medical Technology (Suzhou) Co., Ltd. held a strategic innovation cooperation signing ceremony and a press conference to announce the research and d...
April 02, 2025 | News
Shanghai Moyom’s Aphranel® MagiCCrystal Becomes First NMPA-Approved CaHA Filler in China
Shanghai Moyom Biotechnology's high-profile brand, Aphranel® MagiCCrystal CaHA Filler, has officially obtained Class III Medical Device ...
March 24, 2025 | Regulatory
NEXTBIOMEDICAL’s Nexsphere-F™ Secures FDA Breakthrough Device Designation for Osteoarthritis Pain Treatment
NEXTBIOMEDICAL CO., LTD, a leading developer of cutting-edge Devices, announced that its groundbreaking product 'Nexsphere-F™,' a resorbable microsph...
March 07, 2025 | News
Lunit Expands AI-Powered Diagnostics in Saudi Arabia with Second HMG Partnership
Lunit , a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced a second agreement with Cloud Solutions, a subs...
March 07, 2025 | News
DeepQure Advances Novel Renal Denervation Technology with Clinical Success in U.S. and South Korea
DeepQure Inc, a pioneering medical device startup, is revolutionizing the treatment of resistant hypertension with its innovative extravascular (laparoscop...
March 04, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News