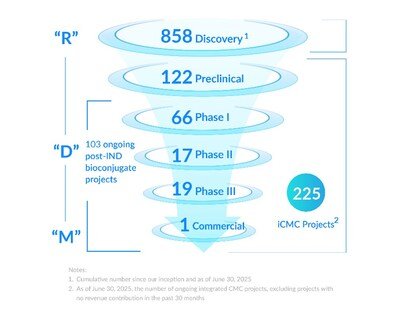
Contract Services Contract Manufacturing
Andelyn Biosciences and Tern Therapeutics Partner to Advance Gene Therapy for CLN2 Batten Disease
Andelyn Biosciences, Inc., a pioneering and patient-focused cell and gene therapy Contract Development and Manufacturing Organization (CDMO), has partnered...
September 08, 2025 | News
China’s WuXi Biologics Secures SBTi Approval for Net-Zero Targets, Reinforcing Global Green CRDMO Leadership
Committed to net-zero across the value chain by 2050 Aligned with 1.5°C mitigation pathways, the most ambitious SBTi designation Leader in Gre...
August 29, 2025 | News
Piramal Pharma Solutions and NewAmsterdam Pharma Expand Global OSD Manufacturing with Facilities in USA and India
Piramal Pharma Solutions, a global leader in contract development and manufacturing (CDMO) and part of Piramal Pharma Ltd. (NSE: PPLPHARMA) (BSE: 543635), ...
August 25, 2025 | News
Andelyn Biosciences Partners with Amplo Biotechnology to Advance AAV Gene Therapies for NMJ Disorders
Andelyn Biosciences, Inc., a leading and patient-focused cell and gene therapy Contract Development and Manufacturing Organization (CDMO), has partnered wi...
August 22, 2025 | News
WuXi Biologics Posts Robust H1 2025 Results with Margin Expansion and Record Project Wins
Revenue increased by 16.1% YoY to RMB 9,953.2 million; revenue growth from continuing operations was 20.2% IFRS Gross profit margin expanded b...
August 20, 2025 | Company results
WuXi XDC Delivers 62% Revenue Growth and Record Project Momentum in H1 2025
- Revenue increased by 62.2% YoY to RMB 2,701 million - Gross profit surged by 82.2% YoY to RMB 975 million, with i...
August 19, 2025 | Company results
GenScript Biotech CEO Sherry Shao Champions Innovation and Global Expansion as H1 2025 Profits Surge Over 500%
GenScript Biotech Corporation (HK.1548), a global pioneer in life sciences innovation, today announced outstanding interim results for the first half of 20...
August 18, 2025 | Company results
WuXi Biologics Achieves Fully Automated Continuous Drug Substance Manufacturing at Pilot Scale with WuXiUP™ Platform
Building on its success in developing continuous production at pilot-scale with the WuXiUP™ platform, WuXi Biologics has further enhanced the ...
August 13, 2025 | News
ProBio Launches GMP AAV Manufacturing Services at New Jersey Facility to Accelerate Gene Therapy Development
ProBio, a leading contract development and manufacturing organization (CDMO) specializing in gene and cell therapy, announced the launch of its cGMP Adeno-...
August 12, 2025 | News
RemeGen Secures FDA Clearance for Phase II Trials of Innovative Bispecific Antibody RC148 in Advanced Solid Tumors
RemeGen Co., announces clearance of IND application by Food and Drug Administration (FDA) for phase II clinical trials for its independently-developed bisp...
August 11, 2025 | News
Axplora Invests €6.5 Million to Expand Vizag Facility as USFDA-Cleared Indian Sites Strengthen Global Strategy
Axplora, a global leader in API small molecule manufacturing, today announced a significant investment at its Vizag site in India to expand production ca...
August 06, 2025 | News
WuXi XDC’s New DP3 Facility Achieves GMP Release, Boosting Global Bioconjugate Manufacturing Capacity
WuXi XDC Cayman Inc. ("WuXi XDC", stock code: 2268.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO) focused on t...
August 05, 2025 | News
AGC Biologics Partners with Valneva to Advance Phase II Shigella Vaccine Supply from Heidelberg Site
AGC Biologics, your friendly CDMO expert, announced a development and manufacturing services agreement with specialty vaccine company Valneva SE ...
July 31, 2025 | News
WuXi Biologics Begins Construction of One of the World’s Largest Modular Biologics Drug Product Facilities in Singapore
Innovative modular design will accelerate construction and manufacturing timelines, enabling rapid adaptation of manufacturing capacities across diverse ...
July 30, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News