Clinical Trials
Enveda Biosciences Secures $55 Million in Latest Financing Round
Enveda Biosciences a biotechnology company using AI to translate nature into new medicines, announced today a new financing round of $55 million to add to ...
June 17, 2024 | News
SciRhom GmbH Secures Approval for Clinical Trial Application of SR-878 in Austria
SciRhom GmbH, a biopharmaceutical company pioneering the development of first-in-class therapeutic antibodies, announced the approval of a clinical trial a...
June 14, 2024 | News
IQVIA Launches One Home for Sites™ to Streamline Clinical Trial Management
IQVIA announced the launch of One Home for Sites™, a new technology platform that acts as a single sign-on and a single dashboard for the key systems...
June 13, 2024 | News
GC Biopharma and Novel Pharma Receive FDA Fast Track Designation for Sanfilippo Syndrome Treatment GC1130A
GC Biopharma (006280. KS) and Novel Pharma have announced that the U.S. FDA has granted Fast Track Designation for their jointly developed MPSIIIA (Sanfili...
June 11, 2024 | News
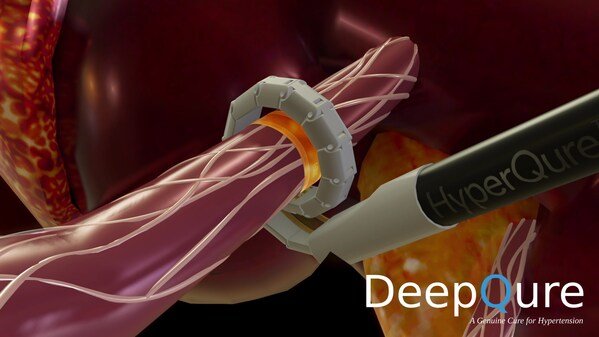
DeepQure Initiates Early Feasibility Study for Groundbreaking HyperQure™ System Following FDA Approval
DeepQure, a Seoul based medical device company with a novel, extravascular (laparoscopic approach) solution for renal denervation (RDN), announce...
June 11, 2024 | News
Vertex's TRIKAFTA® Shows Significant Clinical Benefits in Cystic Fibrosis Patients with Rare Mutations
Results from a randomized, placebo-controlled study of TRIKAFTA® in people with cystic fibrosis with rare, non-F508del CFTR mutations s...
June 10, 2024 | News
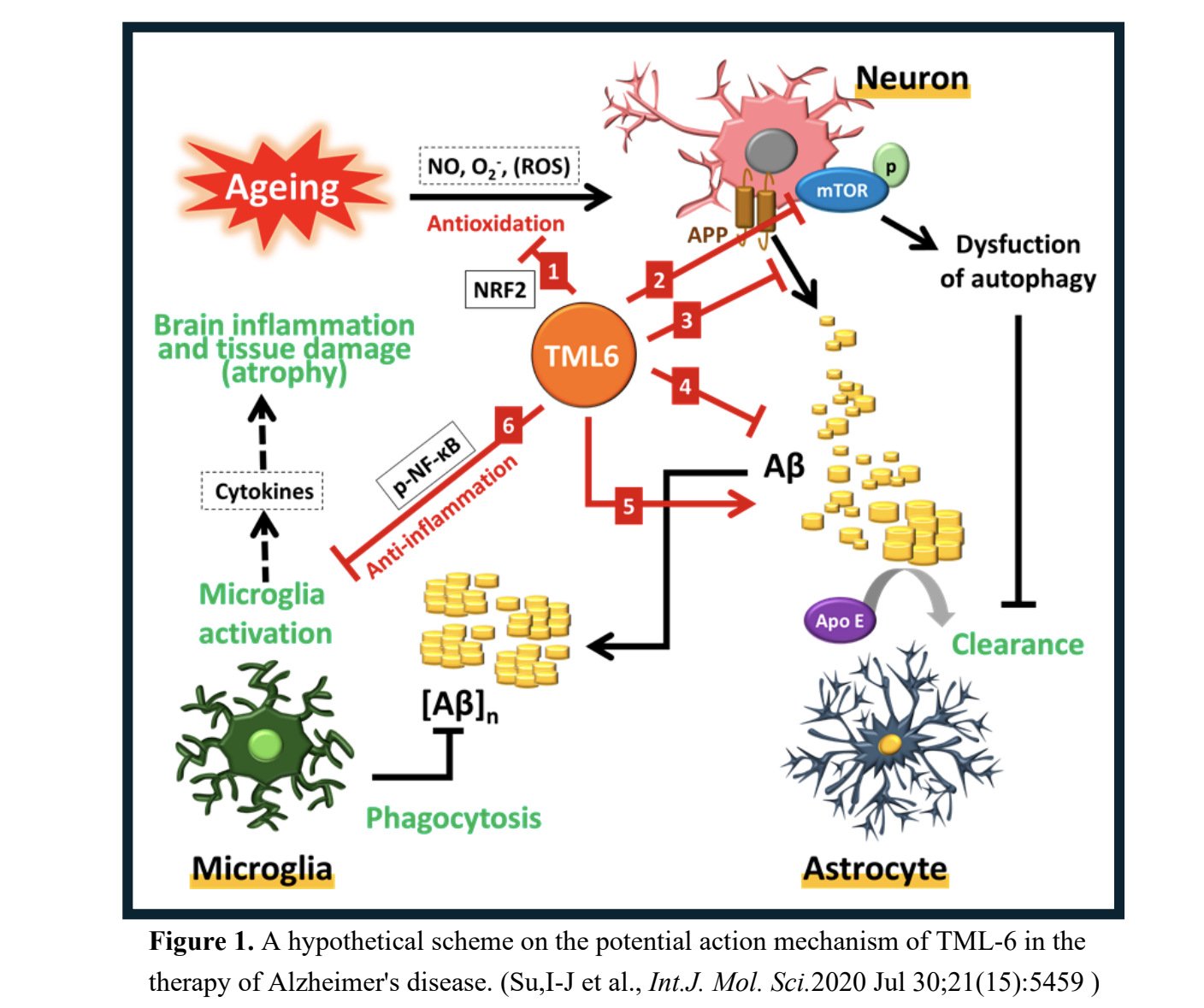
MLB Receives FDA Approval for TML-6 IND Application to Treat Alzheimer's Disease
MLB (Merry Life Biomedical Company, Ltd., Taiwan ) a biomedical company, announced that the U.S. Food and Drug Administration (FDA) h...
June 07, 2024 | News
Shanghai MicuRx Achieves Key Milestone in Combatting Drug-Resistant Infections with Successful Phase I Trial of MRX-8
According to the World Health Organization (WHO), millions of patients face treatment difficulties or even life-threatening conditions due to resistant b...
June 07, 2024 | News
I-Mab Partners with Bristol Myers Squibb for Phase 1 Clinical Trial of Novel Cancer Therapy
I-Mab , a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapi...
June 06, 2024 | News
Thermo Fisher Scientific Opens Cutting-Edge Ultra-Cold Facility in EU to Boost Advanced Therapy Development
Thermo Fisher Scientific Inc., the world leader in serving science, has opened a new clinical and commercial ultra-cold facility in the EU, expanding its c...
June 03, 2024 | News
AstraZeneca’s TAGRISSO® Shows Significant Improvement in Progression-Free Survival in Phase III LAURA Trial for EGFR-Mutated NSCLC
Positive results from the LAURA Phase III trial showed AstraZeneca’s TAGRISSO® (osimertinib) demonstrated a statistically significant a...
June 03, 2024 | News
EMA CHMP Recommends Approval of Sugemalimab for First-Line Treatment of NSCLC
EMA CHMP recommendation is based on the results of a Phase 3 clinical trial (GEMSTONE-302) demonstrating significant progression-free survival (PFS) and ...
June 03, 2024 | News
Fortrea Launches Comprehensive Solution to Enhance Diversity and Inclusion in Clinical Trials
Fortrea, a leading global contract research organization (CRO), today announced its comprehensive and integrated solution to improve the diversity an...
May 31, 2024 | News
Kexing Biopharm’s Subsidiary Receives Approval for Clinical Trials of Innovative GB08 Growth Hormone
Kexing Biopharm announced that Shenzhen Kexing Pharmaceutical Co., Ltd., its wholly-owned subsidiary, recently received a Notice of Approval for Drug Clini...
May 31, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News