Clinical Trials
Ascentage Pharma’s Lisaftoclax NDA Accepted for Priority Review in China: A Potential First for Bcl-2 Inhibitors
Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing and commercializing therapies to address global unm...
November 18, 2024 | News
Medidata and Bioforum Expand Partnership to Enhance AI-Driven Clinical Trials for Biotech Clients
-Medidata, a Dassault Systèmes brand and a leading provider of clinical trial solutions for the life sciences industry announced a new corporate agr...
November 18, 2024 | News
Mabwell's Nectin-4 Targeting ADC Secures IND Approval for Clinical Trials in Perioperative Urothelial Carcinoma and Advanced Solid Tumors
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its novel Nectin-4 targeting ADC (R&D code: 9MW...
November 15, 2024 | News
Perth’s Lixa Kicks Off Clinical Trial for NeoX-101, Aiming to Combat Chronic Lung Infections
Lixa, a Perth-based biotechnology company, has announced the launch of its first clinical trial to evaluate the safety and tolerability of NeoX-101, an inn...
November 14, 2024 | News
Nxera Pharma Secures $3.5M Milestone as Centessa Pharmaceuticals Launches Phase 2 Trial of ORX750 for Sleep Disorders
Nxera Pharma Co., Ltd. (“Nxera” or “the Company”; TSE 4565) – formerly known as Sosei Group or Sosei Heptares – notes t...
November 14, 2024 | News
Transgene and NEC Unveil Positive 24-Month Data for TG4050 Cancer Vaccine in Adjuvant Treatment of Head and Neck Cancer at SITC 2024
Transgene (Euronext Paris: TNG), a biotech company that designs and develops virus-based immunotherapies for the treatment of cancer, and NEC Cor...
November 14, 2024 | News
Taiwan's Caliway Biopharmaceuticals Receives EMA Orphan Drug Designation for CBL-514 in Dercum’s Disease Treatment
CBL-514 is the first and only drug to receive EMA Orphan Drug Designation for Dercum's disease treatment.- CBL-514 received both FDA Orphan Drug...
November 14, 2024 | News
Ascletis Completes Enrollment for Phase III Trial of ASC40 in Acne
Phase III trial enrolled a total of 480 patients with moderate to severe acne-Topline results expected in the second quarter 2025 Ascletis Pharma Inc. ann...
November 13, 2024 | News
Alphamab Oncology’s JSKN033 Receives Fast-Track Approval from Shanghai Drug Authority for Innovative Cancer Therapy Clinical Trials
Alphamab Oncology (stock code: 9966.HK) announced that a clinical trial (Study ID: JSKN033-102) of JSKN033, a high-concentration subcutaneous co-formulatio...
November 13, 2024 | News
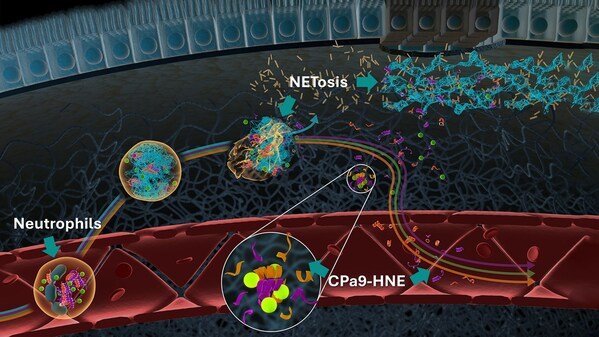
Nordic Bioscience Secures FDA Letter of Support for CPa9-HNE Biomarker in Advancing IBD Clinical Trials
Nordic Bioscience, a leading biomarker company, announced that its CPa9-HNE biomarker assay received a Letter of Support (LoS) from the U.S. Food and Drug ...
November 12, 2024 | News
China’s Gan & Lee’s GZR18 Injection Achieves Up to 17.8% Weight Loss in Phase 2b Trial
Obese or overweight participants receiving bi-weekly doses of 12 mg, 18 mg, 24 mg, 48 mg, and once-weekly dose of 24 mg GZR18 for 30 weeks achieved mean ...
November 12, 2024 | News
Harbour BioMed Files IND Application in China for Novel Antibody Therapy HBM9378/SKB378 Targeting COPD
Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel ant...
November 11, 2024 | News
Dizal Submits NDA to U.S. FDA for Sunvozertinib as a New Treatment Option for Advanced NSCLC with EGFR Exon 20 Insertion Mutations
Dizal (SSE:688192), a biopharmaceutical company committed to developing novel medicines for the treatment of cancer and immunological diseases, announ...
November 11, 2024 | News
Aditum Bio and Leads Biolabs Launch Oblenio Bio to Advance First-in-Class Tri-Specific Antibody for Autoimmune Diseases
Aditum Bio and Leads Biolabs announced the formation of Oblenio Bio, which is being formed in conjunction with an exclusive option and license agreeme...
November 08, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News