China
WuXi Biologics and Medigene Forge Strategic Partnership to Advance TCR-Guided T Cell Engagers for Cancer Treatment
Partnership leverages Medigene's leadership for T cell receptor (TCR) generation and characterization and WuXi Biologics' unique anti-CD3 mAb, its T cell...
August 09, 2024 | News
Sanyou Bio and JiAnTeBo Forge Strategic Partnership to Innovate Metabolic Disease Therapies
Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. (hereinafter referred to as "Sanyou Bio") and Guangdong JiAnTeBo Biotechnology Co., Ltd. (hereinafter r...
August 08, 2024 | News
WuXi Biologics Secures EMA GMP Certification for Five Facilities in China
WuXi Biologics, a leading global Contract Research, Development and Manufacturing Organization (CRDMO), today announced that four manufacturing facilities ...
August 02, 2024 | News
JelloX Biotech Collaborates with Mayo Clinic to Enhance 3D Digital Imaging and AI Analysis for Precision Cancer Diagnosis
JelloX Biotech Inc. ('JelloX') is pleased to announce that it has entered into a collaboration through a know-how agreement with Mayo Clinic to f...
August 01, 2024 | News
Akeso's Ivonescimab sBLA Accepted by NMPA for First-Line Treatment of PD-L1 Positive NSCLC
Akeso (9926.HK) is delighted to announce that the supplemental biologics license application (sBLA) for its independently developed, world's first-in-class...
July 30, 2024 | News
IASO Bio Receives Second FDA IND Approval in 2024 for Groundbreaking Cell Therapy Eque-cel to Treat Multiple Sclerosis
IASO Biotechnology ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therap...
July 25, 2024 | News
IVDCHINA 2024: Premier International Exhibition on In Vitro Diagnostics Set for August in Shanghai
The highly anticipated IVDCHINA 2024, an international exhibition and conference focused on the rapidly evolving field of in vitro diagnostics (IVD), is se...
July 22, 2024 | News
Rona Therapeutics Secures $35 Million Series A+ Financing to Advance siRNA Programs
Rona Therapeutics, a clinical stage pioneering platform company in nucleic acid drug research and development, today announced the completion of $35 M...
July 22, 2024 | News
Everest Medicines Doses First Chinese Patient in Global Phase 2b Trial of Zetomipzomib for Lupus Nephritis
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing an...
July 22, 2024 | News
Everest Medicines' NEFECON® sNDA Accepted by China’s NMPA, Paving the Way for First Full Approval of IgA Nephropathy Treatment
Everest Medicines, a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeuti...
July 18, 2024 | News
Sanyou Bio and Huadong Medicine Advance SYHD001 ADC Drug to Phase I Clinical Trials
Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. (hereinafter referred to as "Sanyou Bio") has recently announced a significant milestone in collaboration wi...
July 17, 2024 | News
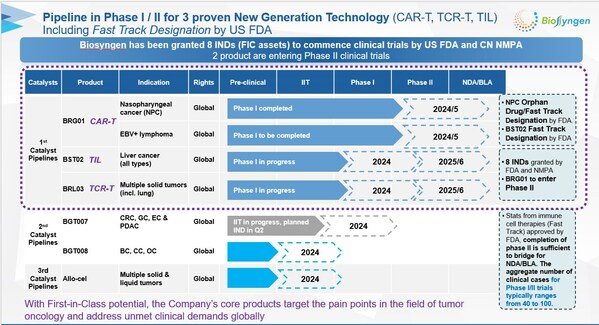
Biosyngen's Groundbreaking CAR-T Therapy BRG01 Approved for Phase II Clinical Trial in China
Biosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation ...
July 17, 2024 | News
Mabwell's Novel Nectin-4 ADC Approved for Phase II Clinical Trial in Treating Triple-Negative Breast Cancer
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821)...
July 16, 2024 | News
Everest Medicines Announces Positive Topline Data from Phase 3 Trial of Etrasimod for Ulcerative Colitis in Asia
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing an...
July 16, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News