China
Sanyou Biopharmaceuticals Introduces Comprehensive Monkeypox Product Line Featuring Antigens, Monoclonal Antibodies, and Overexpression Cell Lines
Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. announced the launch of a comprehensive product line targeting monkeypox, which includes antigens, monoclona...
September 02, 2024 | News
Jacobio Pharma Grants China Rights for KRAS G12C and SHP2 Inhibitors to Shanghai Allist in Landmark Deal
Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, today announced that it has granted the China ...
September 02, 2024 | News
UTime Signs NDA for Strategic Acquisition of Bowen Therapeutics Lab to Boost Global Vaccine Development
UTime Limited has officially announced the signing of a non-disclosure agreement (the "NDA") with Bowen Therapeutics Inc ("Bowen Therapeutics") for th...
August 29, 2024 | News
GenScript’s Legend Biotech Secures NMPA Approval for Pioneering Cilta-cel Therapy in China
Legend Biotech (NASDAQ: LEGN), a subsidiary of GenScript Biotech Corporation (hereinafter referred to as "GenScript"), a global leader in life sciences res...
August 28, 2024 | News
ArkBio CEO Presents Promising IPF Drug AK3280 at 8th Annual IPF Summit in Boston
Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio"), a privately held, clinical-stage biopharmaceutical company today announced that Dr. Jim Wu, Chief...
August 27, 2024 | News
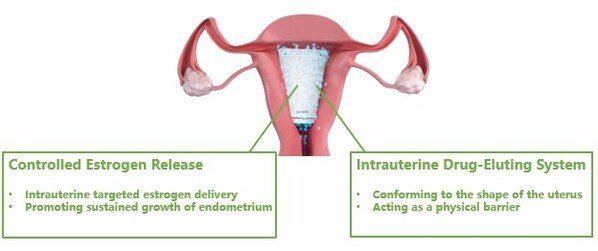
Yipurun's Corina System Receives Approval in China for Treating Moderate-to-Severe Intrauterine Adhesions
Corina Intrauterine Drug-Eluting System ("Corina"), developed by Yipurun (Shanghai) Biotechnology Co., Ltd. ("YPR"), has received approval i...
August 26, 2024 | News
Everest Medicines Launches First Clinical Trial for Personalized mRNA Cancer Vaccine EVM16 in China
Everest Medicines , a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeut...
August 22, 2024 | News
Kelun-Biotech's NDA for Sacituzumab Tirumotecan Accepted by China's NMPA Following Positive Lung Cancer Study Results
New drug application (NDA) for the core product sacituzumab tirumotecan (sac-TMT, formerly SKB264/MK-2870) based on the positive results from the pivotal O...
August 21, 2024 | News
CARsgen Therapeutics Completes Enrollment in Pivotal Phase II Trial of Satri-Cel for Advanced Gastric Cancer in China
CARsgen Therapeutics Holdings Limited , a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors...
August 20, 2024 | News
Astellas Pharma Gains NMPA Approval for PADCEV™ in China to Treat Advanced Urothelial Cancer
Astellas Pharma Inc. (TSE:4503, President and CEO: Naoki Okamura, "Astellas") announced that the Center for Drug Evaluation (CDE) of C...
August 20, 2024 | News
Vimgreen Pharmaceuticals Completes Enrollment for Phase 2 Trial of Novel A2A Receptor Antagonist in Parkinson's Disease
Vimgreen Pharmaceuticals, a science-driven pharmaceutical company focused on the modulation of adenosine signaling, announced the completion of enrollment ...
August 16, 2024 | News
Mabwell's Nectin-4 Targeting ADC Receives Breakthrough Therapy Designation in China for Urothelial Carcinoma
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821)...
August 13, 2024 | News
MSD to Acquire Curon Biopharma’s Investigational Bispecific Antibody CN201 in $1.3 Billion Deal
Merck (NYSE: MRK), known as MSD outside of the United States and Canada, and Curon Biopharmaceutical (Curon), a privately held biotechnology company, annou...
August 12, 2024 | News
Pioneering German Delegation, Led by Stem Cell Expert Prof. Mike Chan, Forges New Era of Sino-German Collaboration in Biotechnology
A landmark German delegation, led by world-renowned stem cell expert Prof. Dato' Sri Dr. Mike Chan, concluded a three-day visit to Baoding, China...
August 12, 2024 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News