BioPharma Drug Approval
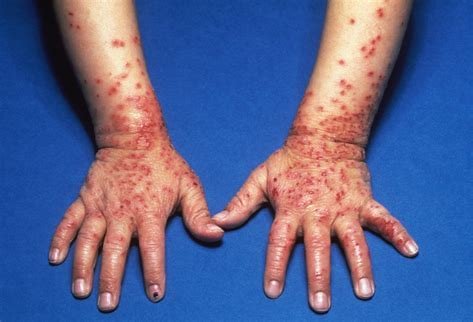
Lynk Pharmaceuticals Announces IND Approval of LNK01004 for Atopic Dermatitis in China
LNK01004 is a novel kinase inhibitor that can simultaneously inhibit multiple cytokine-induced signaling pathways in vitro and in vivo. LNK01004 is tissue-...
August 04, 2022 | News
Armata Pharmaceuticals Announces Clearance of IND for Prosthetic Joint Infections
Second indication for AP-SA02 which targets Staphylococcus aureus including MRSA Potential to significantly improve PJI patient outcomes Expands Armata...
August 03, 2022 | News
Polpharma Biologics Announces FDA File Acceptance for Review of Natalizumab, First Proposed Biosimilar to Tysabri®
The application is for an intravenous (IV) route of administration, with the same dosing regimen, presentation and indication as the reference medicine, Ty...
July 26, 2022 | News
Daewoong Pharmaceutical Gets First Korean US FDA Fast-Track for New Idiopathic Pulmonary Fibrosis Drug
As many Korean pharmaceutical manufacturers are currently attempting to develop new drugs for idiopathic pulmonary fibrosis, Daewoong Pharmaceutical's new ...
July 21, 2022 | News
China CDE Grants Breakthrough Therapy Designation (BTD) to Neurophth's NR082 in LHON
NR082 (rAAV2-ND4), a recombinant adeno-associated viral vector, serotype 2, containing human ND4 codon-optimized gene under the control of the cy...
July 18, 2022 | News
Singapore Based SCG Cell Therapy Announces U.S FDA Clearance
ingapore-based SCG Cell Therapy Pte Ltd ("SCG") announced that U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) ...
July 12, 2022 | News
Brii Biosciences Announces Commercial Launch of its Amubarvimab/Romlusevimab Combination Therapy for COVID-19 in China
Brii Bio progressed the combination therapy from discovery to global Phase 3 data readout and first regulatory approval by the China NMPA in les...
July 07, 2022 | News
Australian developed VIRALEZE™ nasal spray relaunched by LloydsPharmacy in the UK
Extensive antiviral testing undertaken at the globally renowned Scripps Research Institute in the United States show that VIRALEZE&trad...
July 01, 2022 | News
GSK's Zejula (niraparib) Tablet approved in Singapore for first-line and recurrent monotherapy maintenance treatment in advanced ovarian cancer
GlaxoSmithKline (GSK) Singapore today announced that Health Sciences Authority has approved Zejula (niraparib) Tablet, an oral, on...
June 29, 2022 | News
Menarini Group and Radius Health Submit New Drug Application to the U.S. FDA for Elacestrant
Intended for potential treatment of ER+/HER2- advanced or metastatic breast cancer patients Priority Review requested; if accepted, anticipate an 8-mont...
June 23, 2022 | News
FDA Recommends EUA of Novavax COVID-19 Vaccine for People Aged 18 Years and Older
If Emergency Use Authorization is granted by the FDA, the Novavax COVID-19 vaccine would become the first protein-based COVID-19 vaccine available in the...
June 09, 2022 | News
Harbour BioMed Announces IND Approval for B7H4x4-1BB Bispecific Antibody
This study will evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary anti-tumor activity of HBM7008 in patients with soli...
June 09, 2022 | News
Made-In-Singapore Cancer Drug ETC-159 Advances In Clinical Trials
A made-in-Singapore cancer drug, ETC-159, has advanced into the dose expansion portion of its Phase 1B clinical trial. The Phase 1B dose expansion st...
June 06, 2022 | News
Merck Advances Development Programs in Oncology Focusing on Novel Mechanisms and Pathways
New data from DNA Damage Response (DDR) inhibitor portfolio inform development path for this promising biology Clinical trials in locally advanced head ...
June 06, 2022 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News