Lumos Receives US FDA Clearance for FebriDx®
04 July 2023 | Tuesday | Regulatory
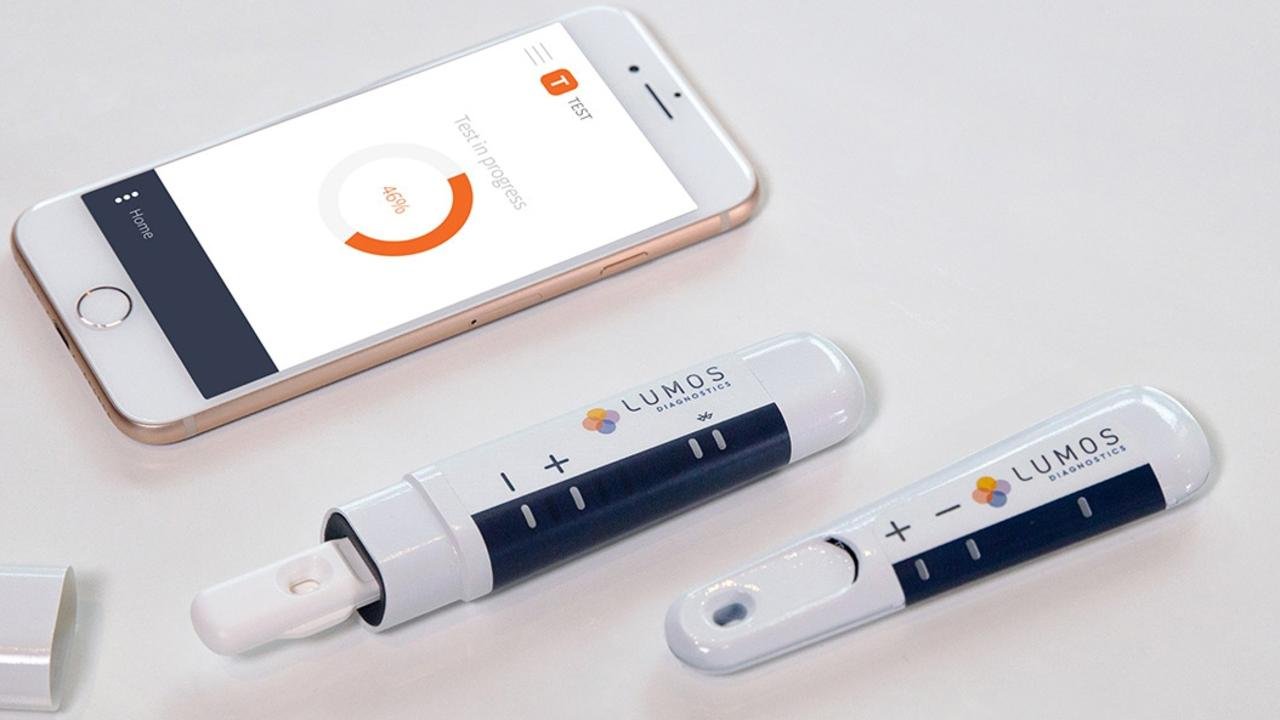
Image Source : Public Domain
The clearance allows FebriDx to be marketed in the US for use by healthcare professionals as an aid in the diagnosis of bacterial acute respiratory infection and differentiation from non-bacterial etiology in patients presenting in urgent care or emergency care settings. FebriDx is intended to be used in conjunction with clinical signs and symptoms, including other clinical and laboratory findings, to evaluate patients for acute respiratory infection.
Following a presubmission meeting in January 2023, Lumos submitted a new 510(k) application for FebriDx to the FDA earlier this year. The FDA has completed its review of this new application and determined that FebriDx has demonstrated substantial equivalence to the predicate device cited in this application, and has consequently cleared it for marketing in the US. FebriDx is already registered in the UK, Europe, Canada, UAE, Brazil, Turkey, Pakistan, Singapore, Malaysia and Australia.
The inappropriate and unnecessary prescribing of antibiotics is recognised as a significant contributing factor to the growing global emergence of antimicrobial resistant (AMR) strains of bacterial pathogens. In 2021, US healthcare professionals in outpatient settings issued 211 million prescriptions for antibiotics—equivalent to 636 prescriptions per 1,000 persons[1]. Despite acute respiratory infections being predominantly viral in origin, they are the most common diagnosis for which antibiotics are prescribed and up to 40% of these prescriptions are considered unnecessary[2]. As a consequence, one of the core elements of the Centers for Disease Control and Prevention (CDC) Outpatient Antibiotic Stewardship program is to improve antibiotic prescribing by clinicians and their use in patients so that antibiotics are only prescribed and used when needed.
"We are delighted to finally secure clearance to market our FebriDx rapid, point–of–care test in the US as we continue to believe it has an important role to play in antibiotic stewardship," said Doug Ward, CEO of Lumos Diagnostics. "It is a credit to the Regulatory team at Lumos that we have been able to deliver this outcome from our new 510(k) application significantly ahead of our initial expectations. With this clearance in hand, we anticipate securing our first commercial orders in the US before the end of calendar year 2023. In the meantime, we are continuing to work with distribution partners and potential licensees, as well as establish our own focused sales effort, as we prepare to launch FebriDx in the US."
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











