MLB Receives FDA Approval for TML-6 IND Application to Treat Alzheimer's Disease
07 June 2024 | Friday | News
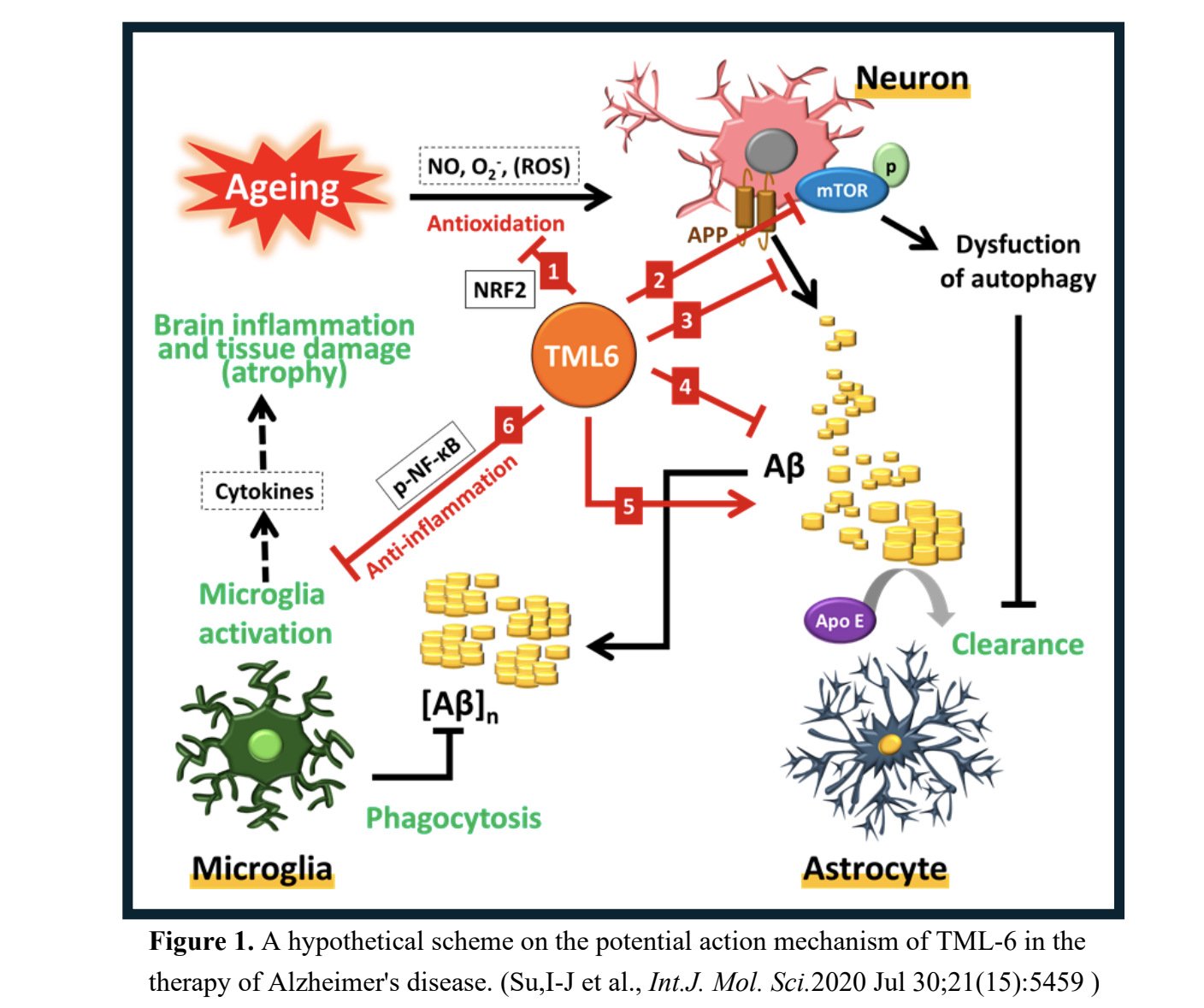
MLB (Merry Life Biomedical Company, Ltd., Taiwan ) a biomedical company, announced that the U.S. Food and Drug Administration (FDA) has approved IND application for TML-6, an novel drug to treat Alzheimer's disease (AD), enabling a Phase 1 clinical trial to be initiated this July. Advancing TML-6 into clinical trial is a critical milestone for MLB to develop a new era multi-target drug for AD since 2018.
TML-6 is a novel synthetic curcumin analog. Professor Ih-Jen Su at Southern Taiwan University used a platform of 6 aginag and AD biomarkers to screen the 12 compounds from Androscience (San Diego, USA) for AD candidate. Preclinical studies showed that TML-6 (ASC-6) exhibits a multi-target action mechanism for AD, including anti-aging, activation of autophagy (mTOR inhibitor), reducing amyloid accumulation, and anti-inflammation (Figure 1), the efficacy confirmed by 2 AD animal models. TML-6 has a high bioavailability through formulation and then completed preclinical toxicology and safety studies. TML-6 should be potentially a novel drug to improve or reverse the progression of early-stage AD.
The Design and Future Plan of TML-6 for AD Clinical Trials
TML-6 is developed as an oral drug and will conduct a SAD/MAD phase 1 clinical trial at Glendale Adventist Medical Center, LA, USA in 2024 Q3. Elderly cohort and CSF pharmacokinetics (PK) studies were specifically designed. For a global multi-site phase 2a clinical trial, several distinguished global AD experts provided consultation. Blood biomarkers will be included in the phase 2a trial as the surrogate endpoint of efficacy. Furthermore, TML-6 is considering to combine with the current anti amyloid drugs in phase 2 trial. The drug combination could not only exhibit synergistic effects to improve AD behavior, reducing amyloid accumulation and ant-inflammation, but can also reduce antibody dosing to only 10% and avoid the adverse events (ARIAs) of anti-body drugs. MLB has successfully raised funds to conduct this global phase 2a clinical trial, scheduled to be conducted on 2025 Q3.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











