Fujitsu and RIKEN develop AI drug discovery technology utilizing generative AI to predict structural changes in proteins
11 October 2023 | Wednesday | News
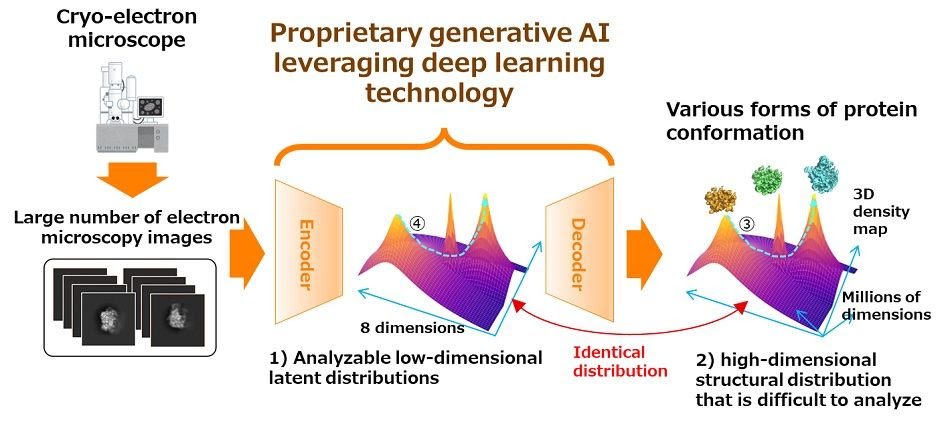
Image: Outline of the newly developed technology Encoder and decoder are trained on images that have been taken in large enough quantities by a microscope. After training, it is possible to obtain an analyzable low-dimensional distribution 1) in the latent space that is equivalent to the structural distribution 2), which is difficult to analyze. At the same time, the decoder can recover various 3D density maps corresponding to low-dimensional features.Future Plans
Fujitsu Limited and the HPC- and AI-driven Drug Development Platform Division of the RIKEN Center for Computational Science today announced that they have developed an AI drug discovery technology that can predict structural changes of proteins from electron microscope images as a 3D density map in wide range by utilizing generative AI in January 2023. The two parties further plan to present a paper on this technology at MICCAI 2023, the top international conference in the field of medical image processing, on October 10, 2023 (Japan time).
In conjunction with this announcement, Fujitsu also plans to make its prediction technology for protein structural changes available on October 10, 2023 as an AI innovation component of the Fujitsu Kozuchi (code name) - Fujitsu AI Platform, an AI platform that allows users to quickly test out advanced technologies.
As part of a joint research project launched in May 2022, Fujitsu and RIKEN developed a generative AI technology that accurately estimates the various forms of a target protein's conformation and their possible proportions from a large number of projection images taken by electron microscopy, as well as a technology that predicts conformational changes in the target protein from the estimated proportions. Based on these two technologies, the two parties developed an AI drug discovery technology that can predict structural changes of a protein in a wide range, with the aim of developing next-generation IT drug discovery technology that significantly reduces the development time and cost of drug discovery.
The technology enables the accurate acquisition of protein conformations and changes based on experimental data in more than ten times less time than conventional procedures (1), thereby enabling innovation in the design process of drugs that bind to target proteins such as bacteria and viruses.
Moving forward, Fujitsu and RIKEN will use the newly developed generative AI technology as one of the core technologies for realizing next-generation IT drug discovery technology that can analyze the complex relationships between target proteins and antibodies, and predict global structural changes of molecules with high accuracy and speed.
Background
Proteins that are closely involved in the lifecycles and disease mechanisms of living organisms are naturally very flexible and interact with other molecules in vivo by changing their structure conformation. For example, to develop drugs that suppress infection by viruses such as COVID-19 that stimulate its infection with conformational changes on their surface proteins, it is necessary to ascertain the various conformational states of the proteins and how they change between conformations. However, conventional structural analysis methods require a high level of expertise and trial and error, demanding considerable time and expenditure to obtain accurate conformational changes. To solve this problem, Fujitsu and RIKEN have developed the following two new drug discovery technologies using generative AI.
Two drug discovery technologies
Fujitsu and RIKEN developed two new drug discovery technologies by utilizing the know-how cultivated through the development of Fujitsu's deep learning technology and applying the knowledge of RIKEN's drug discovery molecular simulation utilizing supercomputer Fugaku (2). The combination of the two technologies reduced the time for prediction of conformational changes in a target protein from one day to two hours (3), thereby contributing to the speedup and efficiency of the drug discovery process for pharmaceutical companies. Details of each technology are as follows:
1. Generative AI technology that accurately estimates the various forms of protein conformation and their proportions
Accurate prediction of conformational changes of a target protein in a wide range requires the possible forms of the conformation and their accurate proportions. In this study, Fujitsu and RIKEN reconstructed a 3D density map of each conformation from a large number of projection images and the corresponding angles at a given moment. At the same time, the two parties estimated the proportion based on the frequency of the reconstructed conformation as a clue.
2. Technology for predicting conformational change based on low dimensional feature of protein conformation
Since the conformation of the target protein is usually expressed by high-dimensional data, it is difficult to directly predict the conformational changes. However, in the process of reconstructing the conformation by the generative AI technology of the preceding paragraph, Fujitsu and RIKEN extracted a low-dimensional feature of the conformation. Using generative AI technology, Fujitsu and RIKEN analyzed the low-dimensional data and predicted the conformational changes by restoring 3D density maps.
Moving forward, Fujitsu and RIKEN will leverage the newly developed AI drug discovery technology as one of the core technologies for analyzing complexes between target proteins and antibodies and for predicting structural changes in molecules with high accuracy and speed. To contribute to the realization of Society5.0 in the field of medicine, RIKEN is promoting the construction of a drug discovery DX platform on supercomputer Fugaku, aiming to innovate the drug discovery process by using it as one of the new technologies to estimate the various structural states of target proteins. RIKEN is further promoting various initiatives including TRIP (4) aimed at creating innovative research platforms that effectively generate new fields of knowledge across research fields. Fujitsu also plans to start offering of its prediction technology for protein structural changes on October 10, 2023 as an AI innovation core component module of Fujitsu Kozuchi (code name) - Fujitsu AI Platform. Under Fujitsu Uvance, which aims to realize a sustainable world, Fujitsu is promoting Healthy Living, which maximizes the life experience of everyone. Fujitsu will continue to contribute to solving social problems in the medical field by developing technologies that combine its strengths in AI and HPC.
(1) Conventional procedure :This refers to the procedure for constructing a sequence of conformational change of a target protein as described in the paper [Kinman et al. (2023)]. In this procedure, the sequence is constructed using existing generative AI, cryoDRGN, which has been trained by a large number of projection images of the target protein.
(2) Supercomputer Fugaku :A computer installed at RIKEN as a successor to the K computer. From June 2020 to November 2021, it ranked first in 4 categories in the supercomputer rankings for 4 consecutive terms. Full operation started on March 9, 2021.
(3) Reduce the time with prediction of a conformational change in a target protein from one day to two hours :The effect of applying commonly used ribosome data to those two technologies. The benchmark time, one day, refers to the running time described in the paper [Kinman et al. (2023)].
(4) TRIP :Transformative Research Innovation Platform of RIKEN platforms
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











