SK bioscience Breaks Ground in Southeast Asia and Southern Hemisphere with 440,000 Dose Vaccine Export to Thailand
21 March 2024 | Thursday | News
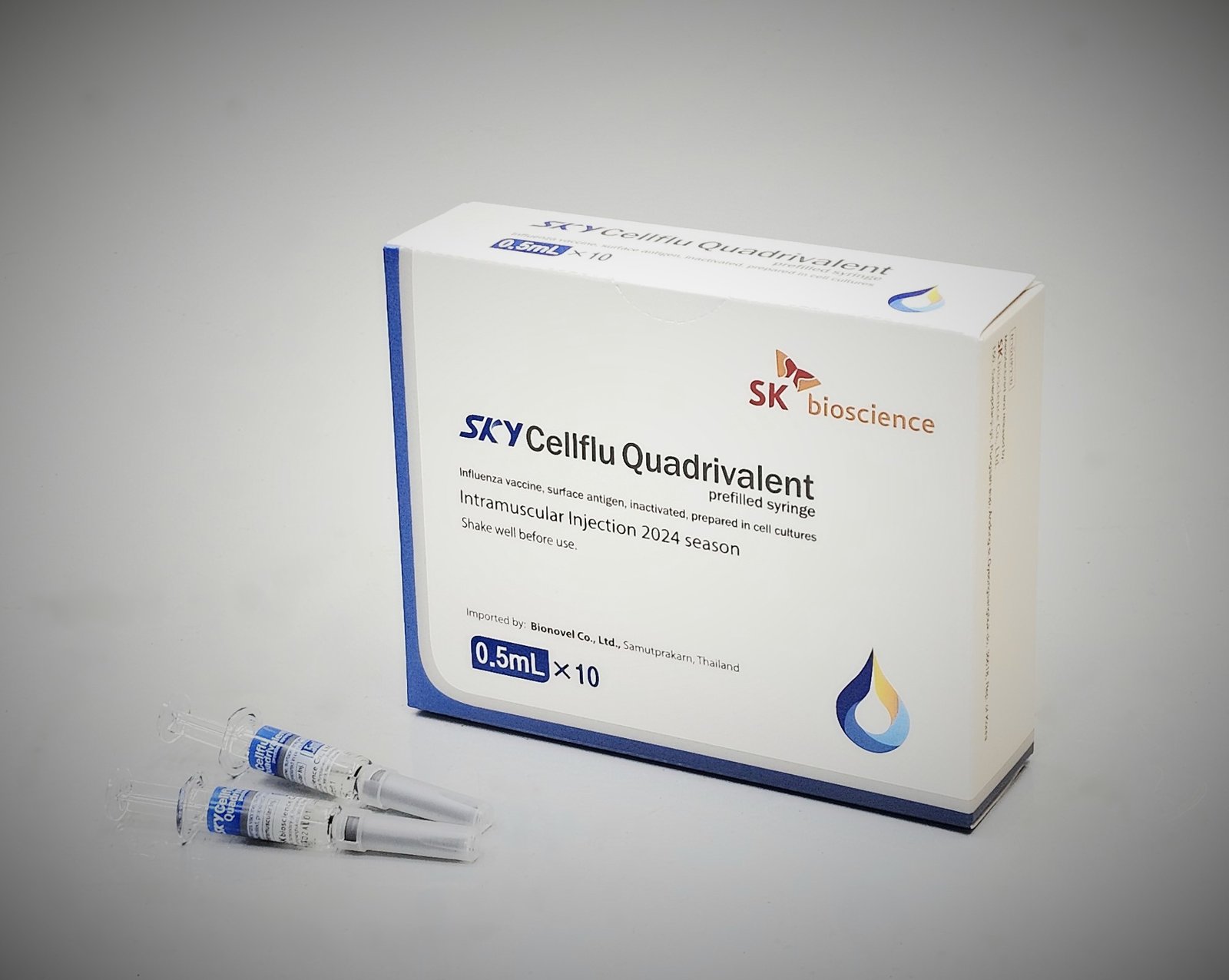
Image Source : Public Domain
- Company exported 440,000 doses to Thailand, marking its entry into the Southeast Asian market and the Southern Hemisphere market.
- Company has obtained marketing authorizations in 12 countries, with additional approvals underway in 10 countries to expand its market worldwide.
SK bioscience, a global innovative vaccine and biotech company committed to promoting human health from prevention to cure, announced today that the company has shipped approximately 440,000 doses of SKYCellflu® to Biogenetech in Thailand from its vaccine manufacturing facility ‘L HOUSE’ in Andong, South Korea.
The vaccine, the company’s self-developed cell-cultured influenza vaccine, contains the recommended composition of influenza virus for use in the 2024 Southern Hemisphere influenza season announced by the World Health Organization (WHO). Starting with the export to Thailand, SK bioscience expects to extend its sales not only in the Southeast Asian market but also in the Southern Hemisphere market.
Thailand is affected by both the WHO's Northern and Southern Hemisphere flu vaccination guidelines due to its elongated geography from north to south. Recently, Thailand Department of Disease Control has recently launched a vaccination campaign aimed at workers in 31 major tourist areas in efforts to prevent influenza after the COVID-19 pandemic.
SKYCellflu® is the world's first cell culture-based influenza vaccine to obtain WHO Pre-qualification (PQ) certification. Last year, the company resumed production of SKYCellflu® and secured first place in the national immunization program bid for the 2023-24 season after a three-year hiatus due to the production of the COVID-19 vaccine during the pandemic.
Compared to the egg-based vaccine, SKYCellflu® is safe for people who are allergic to eggs and does not require antibiotic or preservative administration. Furthermore, it is more suitable for rapid production in response to pandemics.
SK bioscience intends to expand its global market through procurement contacts with international organizations such as the United Nations Children's Fund (UNICEF) and the Pan American Health Organization (PAHO). SKYCellflu® has already received marketing authorizations in 12 countries globally, including Malaysia, Singapore, Mongolia, Pakistan, and Chile. It is currently or will soon be approved in ten more countries, clearing the path for a full-fledged expansion of the export market.
According to Grand View Research, the global influenza vaccine market is projected to expand to approximately $12.58 billion by 2030, with an average annual growth rate of 6.98 percent.
Jaeyong Ahn, CEO of SK bioscience, said, "The export of SKYCellflu® to Thailand serves as a stepping-stone for entering the Southeast Asian and Southern Hemisphere markets and expanding into the global market." He added, " In addition to diversifying our existing product markets, we are committed to developing vaccines with blockbuster potential, such as our next-generation pneumococcal conjugate vaccine candidate, which is expected to enter phase III clinical trials this year.”
Meanwhile, SK bioscience continues to push ahead to explore new sales channels by obtaining regulatory approvals for its self-developed vaccines globally. SKYTyphoid®, its typhoid conjugate vaccine, has received WHO PQ certification, while SKYVaricella®, its varicella vaccine, has obtained marketing authorization in Mexico.
Furthermore, the company is accelerating its ‘Globalization’ project, which transfers its R&D and production capability to other countries to establish region-specific manufacturing hubs for stable global supply. Last July, the company signed a Memorandum of Understanding (MoU) with the Government Pharmaceutical Organization (GPO), a state-owned pharmaceutical company, to transfer manufacturing technology for influenza vaccine. Cooperation discussions are currently underway with countries in Africa, Southeast Asia, the Middle East, Latin America, and Eastern Europe
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











