CorestemChemon Phase 3 ALSummit Trial Shows Subgroup Benefit of Neuronata-R® in ALS
22 September 2025 | Monday | News
CorestemChemon Inc. announced that it has presented key findings from the Phase 3 ALSummit trial of Neuronata-R® (lenzumestrocel) an autologous bone marrow-derived mesenchymal stem cell (MSC) therapy for amyotrophic lateral sclerosis (ALS) at the PACTALS 2025 congress in Melbourne Australia.
The first session chaired by Professor Seung-Hyun Kim of Hanyang University Hospital — a global leader in stem cell-based ALS research — brought together a panel of seven distinguished ALS experts from six countries. Dr. Ryung-A Lee Head of R&D Innovation at CorestemChemon introduced the company and shared an overview of the Phase 3 trial results. The full Phase 3 clinical trial (ALSummit) data were then presented by Professor Ki-Uk Oh principal investigator and neurologist at the Advanced Regenerative Medicine Center Hanyang University Hospital.
Subgroup Analysis Demonstrates Statistically Significant Benefit
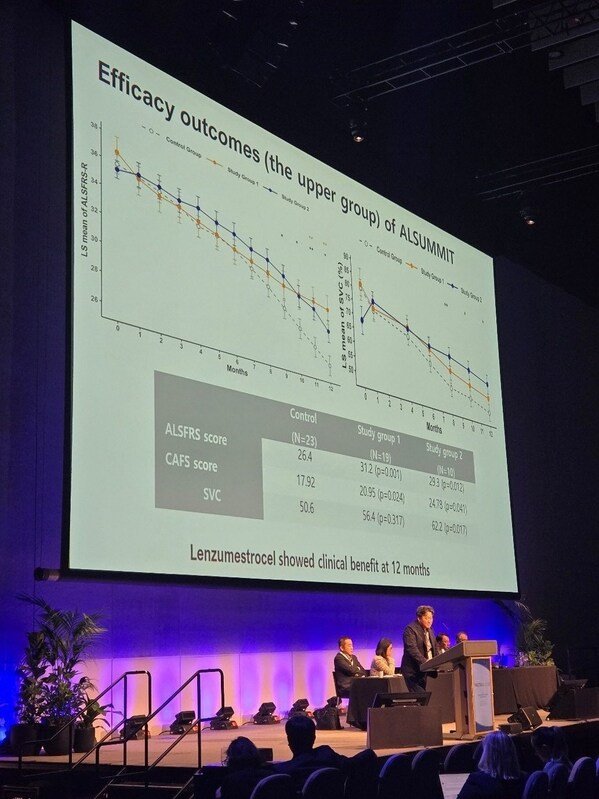
While the overall trial did not meet its primary and secondary endpoints Neuronata-R® demonstrated meaningful efficacy in patients with slower disease progression. At 12 months participants in this subgroup receiving Neuronata-R® achieved:
-
ALSFRS-R (functional rating scale): 31.2 vs. 26.4 in placebo (p=0.001)
-
CAFS (Combined Assessment of Function and Survival): 20.95 and 24.78 vs. 17.92 in placebo (p=0.024; p=0.041)
-
SVC (slow vital capacity respiratory function): 62.2% vs. 50.6% in placebo (p=0.017 Study Group 2)
These findings confirm Neuronata-R®’s potential to preserve function and respiratory capacity in ALS — outcomes directly linked to patient quality of life and survival.
Biomarker Data Underscore Neuroprotective Effect
Biomarker analysis revealed sustained reductions in neurofilament light chain (NfL) and MCP-1 suggesting a strong link between neuroprotection and clinical outcomes. This supports biomarker-guided patient stratification and a precision-medicine approach to ALS therapy aligned with evolving regulatory expectations.
Regulatory Pathway Toward Accelerated Approval
CorestemChemon stated that the PACTALS 2025 results mark an important milestone for regulatory discussions. The company plans to request a Type-C meeting with the U.S. Food and Drug Administration (FDA) in Q4 2025 to review the dataset and subgroup efficacy. Based on this dialogue CorestemChemon expects to submit a Biologics License Application (BLA) in 2026 targeting an accelerated approval pathway consistent with precedents such as FDA’s decision on Tofersen.
Innovative Stem Cell Therapy
Neuronata-R® is designed to address the complex mechanisms of ALS by leveraging MSCs derived from the patient’s own bone marrow. These cells exert anti-inflammatory and immunomodulatory effects protect motor neurons and through paracrine signalling secrete trophic factors cytokines and extracellular vesicles that modulate the microenvironment and reduce neuroinflammation. By targeting these pathological processes Neuronata-R® aims to slow disease progression and improve outcomes for patients with ALS.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











