Skyhawk’s SKY-0515 Shows 62% Reduction in Mutant Huntingtin at Day 84 in Huntington’s Disease Trial
18 September 2025 | Thursday | News
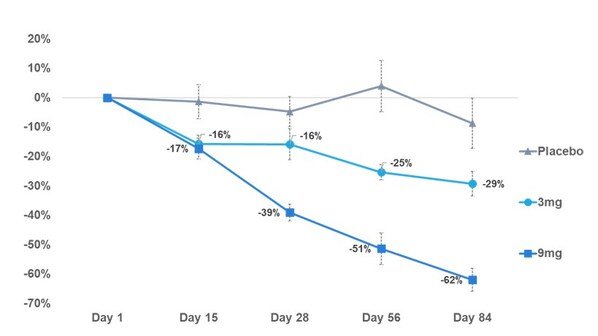
SKY-0515 achieves dose-dependent reductions of mutant huntingtin (mHTT) protein, with 62% lowering at Day 84 on the 9mg daily oral dose
Additional findings include dose-dependent reductions in PMS1 mRNA, excellent brain penetration, and a favorable safety profile
SKY-0515's Phase 2/3 FALCON-HD trial in patients in Australia and New Zealand is ongoing
Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies designed to modulate critical RNA targets, announced positive results from the first interim analysis of the Part C patient cohort in its Phase 1 clinical trial of SKY-0515, an investigational treatment for Huntington's disease (HD). At Day 84, patients receiving SKY-0515 demonstrate dose-dependent reductions of mHTT protein in blood including 62% at the 9mg dose. SKY-0515 has been generally well tolerated at both dose levels tested.
Reduction in mHTT protein level in blood from pre-dose through 84 days of treatment. -- Skyhawk Therapeutics, Inc. ‘Phase 1 Part C patient cohort reduction in mHTT protein level in blood at Day 84,’ 2025; Note: Error bars represent standard error of the mean. Placebo (N=6), 3mg (N=10), and 9mg (N=10) patient treatment is ongoing.
Treatment with SKY-0515 also results in important dose-dependent PMS1 mRNA reduction and demonstrates excellent central nervous system penetration. The overall safety profile has been favorable, supporting the continued clinical development of the program.
"SKY-0515 is reducing mHTT protein to the most impressive extent we've seen so far in patients, and crucially the clinical and biomarker data show no safety concerns so far at any dose tested," said Ed Wild, Professor of Neurology at University College London. "It is great that we are seeing substantial PMS1 reduction as well, which should be a potent combination for treating Huntington's disease via two of its core pathogenic mechanisms. This is what success looks like at the 3-month timepoint, setting the stage for meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 would be truly transformative."
"The strength of SKY-0515's biomarker response after just 84 days of treatment underscores its potential as a transformative therapy for HD," said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics. "These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk's platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies."
Huntington's disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is Skyhawk's orally-administered, investigational small molecule RNA splicing modifier developed through the company's novel RNA-splicing platform, SKYSTAR®. SKY-0515 is designed to reduce both HTT protein and PMS1 protein, an additional key driver of somatic CAG repeat expansion and HD pathology.
SKY-0515 was developed through Skyhawk's proprietary integrated platform, SKYSTAR®, and is the first Skyhawk drug in clinical trials.
Skyhawk expects to put a series of additional novel drugs to treat rare neurological diseases with no presently approved disease modifying therapies in the clinic by the end of 2027. The first is on track to initiate trials in mid-2026.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











