Field Medical's FieldForce™ Ablation System Secures FDA Breakthrough Status
06 December 2024 | Friday | News
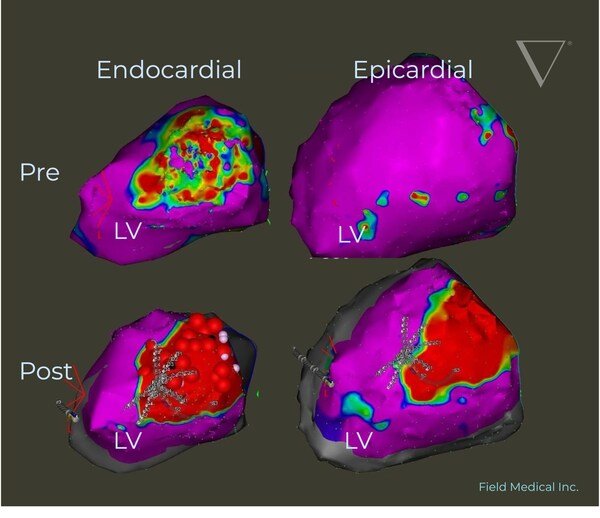
Pre and post voltage map demonstrating Field Medical’s technology creating transmural LV ablation lesions from endocardial treatment alone. This image is from a VCAS Study subject treated with FieldForce for VT Ablation.
- First and only contact force pulsed field ablation (PFA) system engineered to revolutionize care for the hundreds of thousands at risk of death from ventricular tachycardia (VT).
- FDA's TAP Pilot and Breakthrough Device Designation are awarded to medical devices that could provide more effective treatment compared to existing approved treatments, reflecting the agency's commitment to expediting patient access to innovative, safe, and effective therapies.
Field Medical® Inc., a leader in pulsed field cardiac catheter ablation technology, announced that its FieldForce™ Ablation System has been accepted into the FDA's Total Product Life Cycle Advisory Program (TAP) Pilot and granted Breakthrough Device Designation for sustained monomorphic scar-related ventricular tachycardia (VT). This recognition underscores the system's groundbreaking approach to addressing life-threatening VT and the critical unmet needs for patients worldwide.
"The FDA's TAP Pilot acceptance and Breakthrough Device Designation for the FieldForce™ Ablation System represent pivotal milestones in our journey to regulatory approval," said Dr. Steven Mickelsen, CEO of Field Medical. "This recognition advances our vision of equipping electrophysiologists with a next-generation, focal PFA tool for fast, accessible VT care."
Dr. Vivek Reddy, Director of Electrophysiology at Mount Sinai Health System, added, "The FDA's recognition of this breakthrough technology underscores the urgent need for innovation in treating complex, life-threatening ventricular tachycardia. Field Medical's ablation system has the potential to redefine VT care for physicians and patients alike."
The FieldForce™ Ablation System is the first and only PFA system specifically designed for ventricular arrhythmia ablation. Catheter ablation has been shown to be superior to medical therapy in patients at risk of sudden cardiac death (SCD) from VT (Sapp et al., 2024). SCD, which accounts for approximately 450,000 deaths annually in the United States alone (Mason et al., 2022) is often attributed to VT. Current pharmacological therapies are limited in efficacy, with 30-50% of patients with VT not responding well to traditional drug treatments (Kircher et al., 2023). The emerging science highlights the pressing need for innovative solutions like the FieldForce™ Ablation System.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











