RenovoRx Strengthens IP Portfolio with 18 Issued and 13 Pending Patents as it Advances TAMP™ Therapy and RenovoCath Commercialization
02 January 2025 | Thursday | News
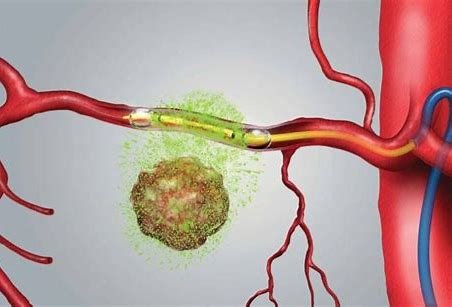
Image Source : Public Domain
RenovoRx Holds a Strong IP Portfolio with 18 Issued Patents and 13 Pending Patents as it Moves Forward with Commercialization and Clinical Trial Plans in 2025
New Patent Filing Anticipated to Expand IP Coverage for RenovoRx’s Innovative TAMP™ Approach to Targeted Drug Delivery
Earlier this year, RenovoRx published a new international patent application for its TAMP therapy platform to expand its IP portfolio. RenovoRx already holds strong IP including coverage of its proprietary TAMP therapy platform and RenovoCath delivery system, with 8 issued and 6 pending U.S. patents, and 10 issued and 7 pending patents outside of the US, including its international application published in 2024.
The new published international patent application WO2024102497 describes methods and tools for delivering treatments (including antibodies, nanoparticles, or charged molecules) directly to specific tissues. This is accomplished using micro-vessels (called the vasa vasorum) that supply the outer walls of larger blood vessels near the specific target tissue to be treated. This invention opens innovative and effective ways to target and deliver cancer treatments in local tissue beyond the current standard-of-care, systemic (intravenous “IV”) treatment.
"Delivering therapeutics through micro-vessels around the artery via our novel Trans-Arterial Micro-Perfusion technique is central to our treatment paradigm and value proposition,” said Ramtin Agah, MD, Chief Medical Officer and Founder of RenovoRx. “We already hold strong IP protection for our technology, and our 2024 pending patent further describes and seeks protection for the novelty of our approach."
TAMP is designed to ensure precise therapeutic delivery across the arterial wall near the tumor site to bathe the target tumor, while potentially minimizing a therapy’s toxicities versus systemic intravenous therapy. RenovoRx’s novel approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy.
"As we move forward in 2025 with our commercial plans for RenovoCath and the advancement of our pivotal Phase III TIGeR-PaC clinical trial, both of which are driven by TAMP, it is important to highlight the strong IP foundation we have and continue to expand. The new patent application filed earlier this year would further the value that TAMP can offer the oncology community as an innovative and effective approach to drug delivery," said Shaun Bagai, CEO, RenovoRx. "Once approved, it will incrementally extend our IP coverage and increase the commercial value proposition for both TAMP and RenovoCath."
RenovoRx’s pivotal ongoing Phase III TIGeR-PaC clinical trial is evaluating its first product candidate, a novel investigational oncology drug-device combination utilizing the FDA-cleared RenovoCath device via TAMP for the intra-arterial administration of the chemotherapy gemcitabine. RenovoRx currently anticipates completion of both patient enrollment and the second interim analysis for TIGeR-PaC by the end of the first half of 2025.
Moreover, RenovoRx recently announced the receipt of its first purchase orders for RenovoCath devices. This milestone marks a positive continuation of RenovoRx’s previously announced efforts to commercialize RenovoCath as a standalone device to be used by doctors in accordance with its FDA-cleared instructions for use. RenovoCath is powered by TAMP.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











