Galux Validates AI-Driven Platform with Atomic-Level Precision in De Novo Antibody Design
23 September 2025 | Tuesday | News
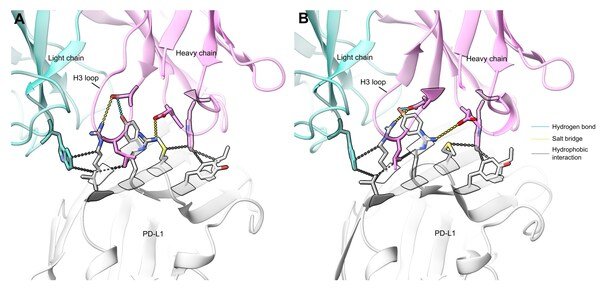
Comparing designed structure and experimentally resolved structure(interface RMSD 1.1 Å)
- Study confirms the novelty and atomic-level precision of de novo antibody design, including cryo-EM validation on a designed anti-PD-L1 antibody
- Findings highlight broad therapeutic applicability across oncology, immune, and metabolic diseases
Galux, a South Korea–based biotech company pioneering AI-driven protein therapeutics design, today announced new results that further validate its de novo antibody design platform, GaluxDesign. The findings showcase the platform's ability to generate novel antibodies that meet precise structural and functional requirements with atomic-level precision across a broad range of targets.
In March, Galux announced antibody designs for six therapeutic targets, at that time one of the most comprehensive demonstrations of de novo antibody design. Those antibodies showed binding affinity, thermal stability, and developability comparable to marketed antibodies, including designs for targets without known structural information.
The new study extends this work to two additional targets, CD98hc and IL-11, bringing the total to eight: PD-L1, HER2, EGFR(S468R mutant), ACVR2A/B, FZD7, ALK7, CD98hc, and IL-11. Notably, IL-11 represents a target without any known antibody complex structure, highlighting the platform's capacity to tackle previously unexplored targets.
For a designed antibody against PD-L1, Galux experimentally determined the antibody–antigen complex using cryo-EM. The designed antibody, GX-aPDL1-3, was highly distinct from all known PD-L1 antibodies in the PDB , sharing at most 43% sequence identity in CDR loops and only 27% in the critical H3 loop. Despite this novelty, the designed structure closely matched the experimentally resolved structures, with an interface RMSD of 1.1 Å, demonstrating atomic-level precision.
The antibodies were initially validated in scFv format and subsequently characterized in IgG format, where favorable stability and binding properties were confirmed. Biophysical analyses across five targets reinforced the robustness of the designs. Notably, subtype- and mutant-specificity observed in scFv was preserved in IgG for antibodies targeting FZD7 and EGFR-S468R, while IL-11 binders successfully targeted multiple biologically meaningful epitopes as intended, demonstrating that binding properties can be rationally tuned.
Beyond the technical achievement of de novo antibody design, the diversity of targets underscores the platform's potential applicability across therapeutic areas. The eight proteins are linked to oncology, fibrosis, metabolic disorders, and immune diseases, suggesting opportunities to address a wide range of therapeutics, including those difficult to target with conventional approaches.
"Our findings indicate that antibodies designed entirely in silico can achieve both novelty and atomic-level precision," said Chaok Seok, CEO of Galux. "We believe this approach can expand the possibilities across diverse disease areas, opening new opportunities for precision molecular design that maximizes efficacy and minimizes toxicity, crucial for successful later-stage development."
Galux is advancing its platform to address previously undruggable targets. The company is also in discussions with global pharmaceutical partners to explore collaborative opportunities.
"We believe this is only the beginning," Seok added. "Our vision is to build a future where medicines are no longer discovered by chance, but rationally and precisely designed: faster, more efficiently, and for patients who need them the most around the world."
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











