See-Mode Technologies' AI-powered Ultrasound Software Secures Regulatory Approval in Australia and New Zealand.
22 March 2023 | Wednesday | News
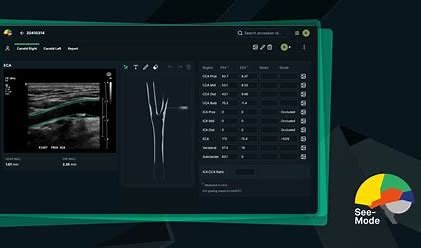
Image Source : Public Domain
"We are thrilled to receive regulatory approvals for our product in Australia and New Zealand," said Dr Milad Mohammadzadeh, Co-Founder and Director of See-Mode Technologies. "Our mission is to provide state-of-the-art medical imaging technology that helps clinicians make faster and more accurate diagnoses, and these approvals bring us one step closer to achieving that goal."
See-Mode's breast and thyroid ultrasound reporting solutions have the potential to revolutionise reporting workflows and improve patient outcomes in Australia and New Zealand," said Dr Milad Mohammadzadeh. "We are excited to partner with leading healthcare providers in the region to bring these benefits to clinicians and patients."
See-Mode uses Artificial Intelligence to detect lesions in ultrasound images, then assigns feature classifications to each lesion, in line with the American College of Radiology's BI-RADS and TI-RADS rating systems. Sonographer worksheets, complete with lesion classifications and diagrams are instantly generated and sent to PACS, while preliminary impressions are sent to radiology reporting systems. In follow-up scans, See-Mode allows for fast comparison between old and new images, and will automatically highlight changes in lesion characteristics.
Studies conducted by See-Mode determined there were large inconsistencies in describing the characteristics of nodules and lesions by sonographers. For Thyroid studies, See-Mode has the potential to reduce unnecessary biopsies and provide clinicians with more confidence around whether or not a biopsy should be performed. With Breast ultrasound examinations, See-Mode assists clinicians to detect lesions that may otherwise go undiscovered or be misclassified.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











