Vetter Introduces Next-Gen V-OVS® Closure System: Integrating Customer Insights for Enhanced Performance
15 January 2025 | Wednesday | News
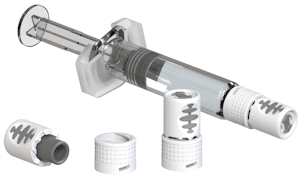
New version integrates valuable real-world learnings and customer feedback
- The latest version of Vetter’s proprietary closure system, V-OVS® next, will build on decades of successful customer applications
- The updated system has been designed to set a new standard of functionality for the syringe-based product market
- Updates to the design, handling, and opening mechanism will further enhance the closure’s performance
Vetter, a globally operating Contract Development and Manufacturing Organization (CDMO), has announced the development and upcoming launch of the new version of its proprietary V-OVS® syringe closure system.
The new closure, V-OVS® next, will further advance a proprietary system with many years of proven market success supporting and differentiating a range of sterile injectables. This latest evolution will integrate several unique technical advances guided by both shifting market needs and feedback from the many customers who have selected V-OVS® for their products. Similar to the original system, the design of V-OVS® next will be optimized to protect the integrity of the injectable product secured by the closure. For customers with products in glass-barrel syringes, this new closure will be an opportunity to integrate gold-standard Luer Lock features with even easier handling characteristics and an even more intuitive opening mechanism.
“With its proprietary tamper-evident features, we’re confident that V-OVS® next will bring a new level of user-friendliness to Luer Lock applications,” says Tobias Nemeth, Director Primary Packaging Service & Projects at Vetter. “It will further build on the decades of market impact delivered by this industry-leading system, and provide a differentiating choice for customers with an even wider range of injectable products.” In addition to handling enhancements, the overall size will also be reduced to better fit smaller syringe sizes. New updated gripping surfaces will provide optimal control during opening, and a robust new locking mechanism will help keep the closure safely in place even during the most challenging clinical use cases.
While still in development, Vetter has already placed V-OVS® next on a launch pathway for 2027. For now, the focus remains on finalizing the system’s design and further advancing its commercialization. As part of its go-to-market preparations, Vetter recently conducted a successful human factors study with a representative group of frontline clinical users. The study delivered consistent positive feedback and insights that will directly contribute to finalizing the design of the new closure system.
Post-launch, Vetter will continue to offer both V-OVS® and V-OVS® next, expanding its portfolio to offer customers more options for their syringe-based products.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











