Breakthrough Discoveries on Novel Features of Omicron Variant and An Anti-Omicron Antibody JMB2002
04 January 2022 | Tuesday | News

Image Source : Public Domain
A team of scientists led by Dr. Su-Jun Deng from biologics of Jemincare R&D Center, and another team of scientists from SIMM of CAS, led by Professor H. Eric Xu and Dr. Wanchao Yin, not only confirmed the binding and pseudovirus neutralization activity of JMB2002 against Omicron variant, but also solved the structures of Omicron spike protein in complex with ACE2 and JMB2002 respectively (Figure 1 and Figure 2). Joint research efforts revealed the mechanisms of increased infectivity and immune escape of the Omicron variant at molecular level, and demonstrated the unique binding mechanism of JMB2002 differing from all reported NAbs. Detailed findings of novel features of Omicron variant and JMB2002 have been published on bioRxiv preprint website (Reference 1).
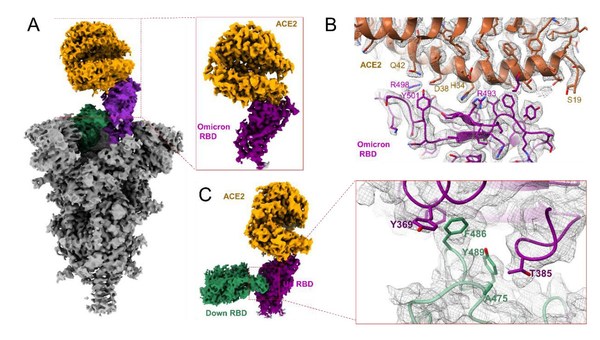
Figure 1. Structure of complex of SARS-CoV-2 Omicron variant spike protein RBD bound to ACE2. A. Overall structure and conformation of ACE2-RBD complex. B. RBD-ACE2 interaction interface. C. The interaction interface of RBD dimer.
The latest research results indicated that JMB2002 had high binding activity to the Omicron variant and showed potent Omicron pseudovirus neutralization function (Figure 2A, 2B). It is encouraging considering that most approved and clinical-stage SARS-CoV-2 neutralizing antibody drugs have lost their neutralization activity or have shown significantly reduced neutralizing potency due to multiple mutations of the spike protein in Omicron variant.
One reason of the enhanced infectivity of Omicron variant is that its spike protein RBD (Receptor Binding Domain) has higher binding ability to the SARS-CoV-2 receptor ACE2 than that of wild type. There are immediate needs for developing specific therapeutic antibodies targeting Omicron variant. Scientists from Jemincare found that the binding affinity of JMB2002 Fab to the spike protein of Omicron variant is 4-fold higher than that of WT (Figure 2A). More importantly, professor H. Eric Xu's group has solved the structure of the complex of Omicron spike trimer bound to JMB2002 (Figure 2C), the structure shows JMB2002 binds to the back of RBD, a unique binding epitope with novel conformation (Figure 2D). It suggests that JMB2002 is a new class of SARS-CoV-2 neutralizing antibody with a binding mechanism different from all reported NAbs, classifying as class V NAb. The results from pseudovirus neutralization assay indicate that JMB2002 is a broad-spectrum neutralizing antibody targeting all WHO VOC except the Delta variant (Reference 1).
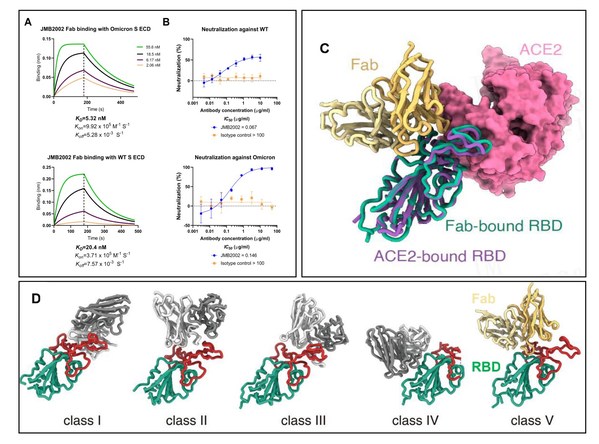
Figure 2. A. Binding of JMB2002 Fab to spike protein of Omicron and WT respectively. B. Neutralizing bioactivities to WT and Omicron variant by JMB2002 in pseudovirus neutralization assay. C. The comparison of the complex of Omicron Spike protein RBD bound to JMB2002 and ACE2. D. Novel binding epitope to Omicron Spike protein by JMB2002, classified as class V neutralizing Ab (NAb)
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











