Singapore Approves Everest Medicines' NEFEGAN® for Adult Primary IgA Nephropathy
20 March 2024 | Wednesday | News
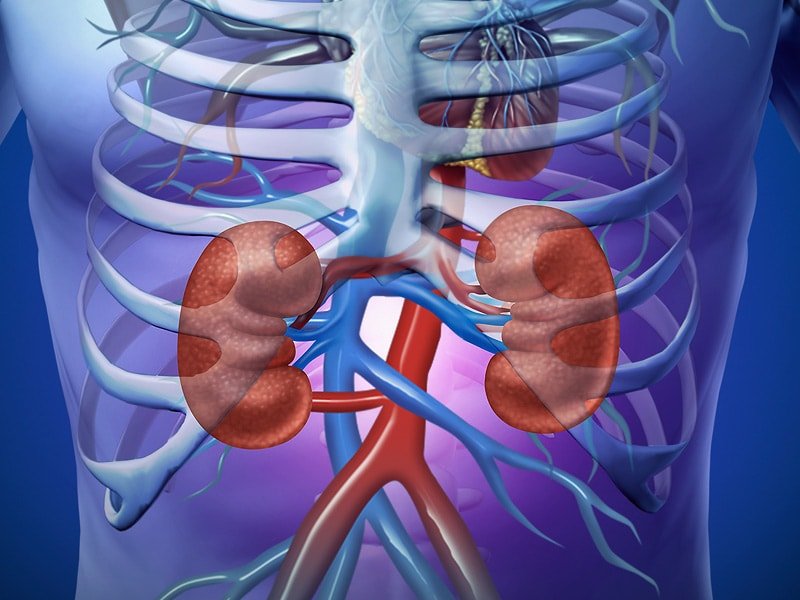
Image Source : Public Domain
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, development, manufacturing and commercialization of innovative medicines and vaccines, announced that the Singapore Health Sciences Authority (HSA) has approved NEFEGAN® for the treatment of primary immunoglobulin A nephropathy (IgAN) in adults at risk of disease progression. NEFEGAN®, known in other Everest territories as Nefecon®, was the first ever treatment for IgAN fully approved by the U.S. Food and Drug Administration, and Singapore marks the third region in Everest territories that received New Drug Application (NDA) approval after Macao and mainland China.
"The NDA approval in Singapore marks an important event for IgAN patients in the region as we continue to expand patient access to Nefecon® throughout Asia, an area of high IgAN disease prevalence," said Rogers Yongqing Luo, Chief Executive Officer of Everest Medicines. "Following approval of Nefecon® in mainland China and commercial launch in Macao, we are working to rapidly expand availability of this first-in-disease therapy which targets the origin of the disease and can slow disease progression to more underserved patients in 2024 including Hong Kong, Taiwan and South Korea."
The Phase 3 NefIgArd clinical trial, a randomized, double-blind, multicenter study, evaluated the efficacy and safety of Nefecon® at a once-daily dose of 16 mg, compared to placebo, in adult patients with primary IgAN on optimized RASi therapy. The NefIgArd study is a 2-year trial, which consisted of nine months of treatment with Nefecon® or placebo, followed by a 15-month follow-up period off study drug. The primary endpoint, time-weighted average of eGFR over 2 years, showed a statistically significant and clinically meaningful benefit of Nefecon® over placebo (p-value < 0.0001). It also showed a difference in 2-year total eGFR slope of 2.95 mL/min per 1.73 m2 per year in favor of Nefecon®. The data reflected treatment benefits across the entire study population, regardless of UPCR baseline level.
The full two-year results of the NefIgArd trial (n=364 patients) were further analyzed to assess potential differences in response to Nefecon® treatment based on self-reported Asian (n=83) or White (n=275) ancestry in patients with IgAN. Treatment with Nefecon® 16 mg/day over a 9-month period resulted in clinically meaningful preservation of kidney function in both subgroups, as evidenced by reduction in proteinuria and stabilization of eGFR in these two subgroups when compared to placebo.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











