The Best of 2022: FDA Approvals and the Breakthroughs That Enabled Them
31 December 2022 | Saturday | News | By Calley Jones, PhD

Image Source : Public Domain
For a field like cancer research, in which progress can sometimes appear to happen slowly, it is especially important to remind ourselves of the crucial advancements being made and the impact they have on patients’ lives. In an article in Cancer Discovery, a journal of the AACR, the AACR Cancer Progress Report 2022 Steering Committee highlighted the key findings of three reports the AACR released this year: the AACR Cancer Progress Report 2022, the AACR Cancer Disparities Progress Report 2022, and the AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. The highlights, also summarized in this blog post, include some heartwarming advances, such as a continued decrease in cancer mortality and a robust increase in cancer survivorship in the U.S. The reports also shed light on the health disparities faced by cancer patients of various racial, ethnic, socioeconomic, and other minorities in the U.S. and emphasize the need for more federal research funding to catalyze critical advancements in cancer care.
The article was published as part of the In Focus section of the December issue of Cancer Discovery. The journal dedicated the section to unique perspectives on the progress made in the past year in areas such as precision oncology, drug development, and genomic data sharing.
In one of the articles in this section, Elizabeth Duke, MD, of the Office of Oncologic Diseases at the U.S. Food and Drug Administration (FDA), and colleagues summarized the key approvals rendered by the FDA for cancer therapeutics in 2022. In another, Debyani Chakravarty, PhD, a molecular geneticist at Memorial Sloan Kettering Cancer Center, and colleagues reviewed the year’s advances in precision oncology and forecasted the priorities for the field in the coming years.
FDA APPROVALS IN 2022
In the year 2022, as of December 30, the FDA has issued 40 new actions related to cancer care, including 28 regular approvals, nine accelerated approvals, two conversions from accelerated to full approval, and three withdrawals of accelerated approvals. Accelerated approval indicates that confirmatory clinical trials may be required to continue marketing the therapy.
The approvals included 12 new therapeutics that span the gamut from targeted therapies to immune checkpoint inhibitors to a new form of CAR T-cells. These therapies are:
- Ciltacabtagene autoleucel (Carvykti) Approved in February, ciltacabtagene autoleucel is a new type of CAR T-cell therapy that targets the protein BCMA. It was approved for adult patients with multiple myeloma whose tumors have relapsed following four or more lines of therapy.
- Tebentafusp-tebn (Kimmtrak) The first oncology FDA approval of the year, tebentafusp-tebn was approved to treat certain adult patients with uveal melanoma, a cancer of the eye. Tebentafusp-tebn is a bispecific antibody that binds to gp100 on cancer cells and CD3 on T cells, bringing the two cell types together to enhance the antitumor immune response.
- Lutetium 177 Lu vipivotide tetraxetan (Pluvicto) This radiotherapeutic, approved in March, targets prostate-specific membrane antigen (PSMA), expressed in many prostate cancers. A companion diagnostic to evaluate prostate tumors for PSMA and determine eligibility for this therapy was approved on the same day.
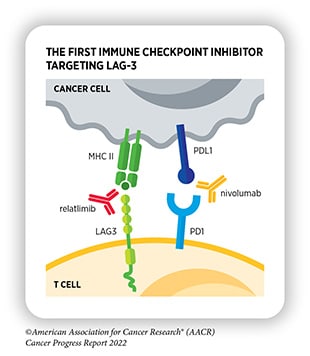
- Nivolumab and relatlimab-rmbw (Opdualag) Also approved in March, this combination therapy consists of two immune checkpoint inhibitors—nivolumab, an antibody that blocks the activity of PD-1, and relatlimab-rmbw, a first-in-class antibody that blocks the activity of LAG-3. It is approved to treat patients aged 12 years and older with unresectable or metastatic melanoma.
- Futibatinib (Lytgobi) This targeted therapy was approved in September to inhibit the fibroblast growth factor receptor 2 (FGFR2) in advanced cholangiocarcinoma found inside the liver.
- Tremelimumab (Imjudo) Approved in October, this immune checkpoint inhibitor is an antibody against the protein CTLA-4. It is approved for use in combination with the PD-L1 inhibitor durvalumab (Imfinzi) for certain adult patients with liver cancer that cannot be surgically removed. The combination was also approved in November for the treatment of metastatic non-small cell lung cancer (NSCLC).
- Teclistamab-cgyv (Tecvayli) This bispecific antibody, approved in October for certain patients with advanced multiple myeloma, binds to BCMA on myeloma cells and CD3 on T cells, which helps the T cells identify and destroy the cancer.
- Mirvetuximab sorvtansine-gynx (Elahere) A first-in-class folate receptor alpha (FRα)-targeted therapy, this antibody-drug conjugate was approved in November to treat adult patients with ovarian, fallopian tube, or peritoneal cancer that is resistant to platinum chemotherapy and expresses FRα.
- Olutasidenib (Rezlidhia) Approved in December for patients with relapsed or refractory acute myeloid leukemia, olutasidenib is a targeted therapy that blocks the activity of IDH1 in patients whose tumors harbor an IDH1 mutation.
- Adagrasib (Krazati) In December, adagrasib became the second FDA-approved drug targeted to a common mutation in KRAS, one of the most frequently mutated genes in human cancer. Adagrasib was approved to treat NSCLC tumors harboring the KRASG12C mutation.
- Nadofaragene firadenovec-vncg (Adstiladrin) The first gene therapy approved to treat bladder cancer, nadofaragene firadenovec was approved in December to treat certain patients with non-muscle-invasive bladder cancer. The therapy is intended for patients with localized tumors that did not respond to the Bacillus Calmette-Guérin vaccine.
- Mosunetuzumab-axgb (Lunsumio) In December, this therapy became the first bispecific antibody approved to treat a form of non-Hodgkin lymphoma. Mosunetuzumab binds to CD20 on follicular lymphoma cells, which helps the T cells identify and destroy the cancer cells.
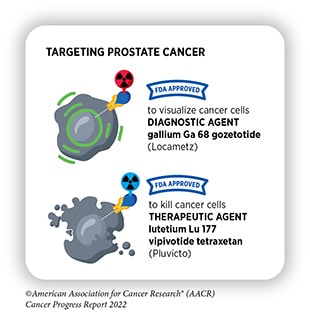
Additionally, Duke and colleagues highlighted notable approvals for new indications of existing drugs. These include two drugs approved for tumor-agnostic use, meaning these drugs can be used in any tumor type as long as the cancer harbors a specific mutation or expresses a specific target. These approvals are often based on data from so-called basket trials, which enroll patients with a variety of cancers.
The combination of dabrafenib (Taflinar) and trametinib (Mekinist)—which was previously approved to treat melanoma, NSCLC, and anaplastic thyroid cancer—received accelerated approval in June for the treatment of any unresectable or metastatic solid tumor in patients aged 6 and older. Dabrafenib inhibits the protein kinase BRAF, which is mutated in 5 to 10 percent of solid tumors overall, with especially high rates in melanoma and colorectal cancer, and trametinib inhibits the protein kinase MEK, a downstream effector of BRAF. The combination is intended for patients whose tumors have the BRAF mutation V600E.
In September, the FDA also issued an accelerated approval to the RET inhibitor selpercatinib (Retevmo) for advanced solid tumors with a rearrangement in the RET gene. On the same day, selpercatinib was granted full approval for the treatment of NSCLC, an indication for which it had previously received accelerated approval in 2020.
The year brought significant advances in pediatric cancer treatment as well. Three drugs were approved for new pediatric indications—azacitidine (Vidaza) for juvenile monomyelocytic leukemia, brentuximab vedotin (Adcetris) for Hodgkin lymphoma, and atezolizumab (Tecentriq), the first PD-L1 inhibitor approved for a pediatric sarcoma.
The oncology approvals for 2022 also included two drugs to reduce toxicity associated with cancer therapy in children. In August, the FDA approved ibrutinib (Imbruvica) for pediatric patients with chronic graft-versus-host disease, a condition in which immune cells transplanted from a donor attack the cancer patient’s healthy tissues. Ibrutinib has been approved since 2017 to control this condition in adults, but it is the first treatment of its kind approved to treat the disease in children.
A month later, the FDA approved sodium thiosulfate (Pedmark) to reduce the risk of hearing loss driven by cisplatin-containing chemotherapy regimens. The drug was approved to be given alongside cisplatin to pediatric patients older than one month of age.
Additional treatments approved this year include therapies for new tumor types or patient populations, therapies greenlit for new lines of treatment, drugs used in new combinations, and a few new formulations or dosing regimens.
BREAKTHROUGHS DRIVING DRUG DEVELOPMENT
The article by Chakravarty and colleagues examined some of the most significant advances in areas such as drug development, clinical trial design, and biomarker testing that made the progress in 2022 possible. With a particular focus on precision oncology, these advances may set the stage for further developments in 2023.
In terms of drug design, Chakravarty and colleagues pointed to cutting-edge breakthroughs in the identification of new drug targets. One example is the GTPase KRAS, well-known for its high prevalence across most cancer types and its tricky structure that previously rendered it “undruggable.” Thorough reassessments of KRAS structure recently led to the design and approval of sotorasib (Lumakras) and adagrasib, which target the KRASG12C mutation commonly observed in lung cancer.
Redefining KRAS as a viable drug target spurred progress for additional investigational KRAS drugs in 2022, including RMC-9805 and MRTX1133, which target KRASG12D; and pan-RAS inhibitors such as RMC-6236.
The success of KRAS inhibitors have also led researchers to reevaluate other supposedly undruggable targets, such as the tumor suppressor p53. Several research groups are currently developing drugs capable of restabilizing p53 proteins harboring the Y220C mutation, which destabilizes the DNA binding domain, rendering the mutant protein ineffective.
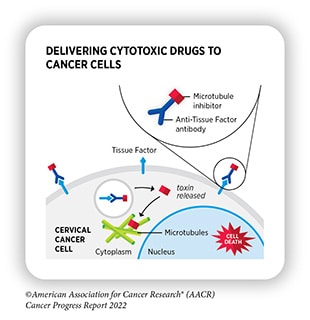
In addition to identifying and leveraging new drug targets, researchers are using emerging therapy types to expand treatment options for tumors with targetable mutations. One example is the growing popularity of antibody-drug conjugates (ADCs)—two-part molecules that bind to a tumor cell, promote cellular internalization of the ADC, and release a cytotoxic drug payload inside the cell.
The ADC trastuzumab deruxtecan (T-DXd), which binds to the growth factor receptor HER2, recently became the first targeted therapy approved to treat breast tumors with low expression of HER2. It was also approved to treat HER2-positive NSCLC, becoming the first HER2-targeted therapy approved for lung cancer.
Brentuximab vedotin, an ADC targeting the protein CD30 on certain lymphoma cells, was also approved in November as part of a combination therapy to treat pediatric patients with Hodgkin lymphoma. This approval came only a few days before a new ADC, mirvetuximab soravtansine-gynx, was approved to target folate receptor alpha in ovarian, fallopian tube, and peritoneal cancers.
Researchers also made strides in 2022 toward creating new small molecules that improve upon their predecessors by enhancing specificity, delaying resistance, or reducing toxicity. Chakravarty and colleagues pointed to two investigational drugs that advanced in development in 2022, both of which had markedly higher affinity for their intended target than other drugs in their class. The EGFR inhibitor CLN-081 had lower cross-reactivity with HER2 than its predecessors and received a breakthrough therapy designation from the FDA this year. Similarly, the ROS1 inhibitor NVL-520 avoids cross-targeting the kinase TRK, which can cause neurological toxicities when inhibited.
Decreasing toxicity is also a priority for researchers working on PI3K inhibitors, which can cause hyperglycemia. One way in which researchers are working to circumvent this issue is by developing inhibitors specific to mutant PI3K, including multi-mutation inhibitors like RLY-2608 and inhibitors targeting a specific mutation, such as STX-478, which targets PIK3CAH1047X, and LOXO-783, which targets PIK3CAH1047R.
Similarly, researchers have also sought to develop new generations of drugs that can overcome mutations associated with therapy resistance. The recently approved FGFR2 inhibitor futibatinib and its close cousin pemigatinib (Pemazyre) are active against a handful of gatekeeper mutations, blocking the most common paths the kinase may use to escape the drug’s effects. New drugs targeting resistance-associated mutations in ROS1/TRK fusions, RET, and ALK are also undergoing active development.
In addition to advances in drug development, Chakravarty and colleagues explained that changes to clinical trial design also helped move research forward in 2022. The increasing prevalence of tumor-agnostic basket trials assisted with the accelerated approvals of selpercatinib and dabrafenib plus trametinib for all solid tumors harboring the drugs’ respective targets. A growing focus on racial and ethnic equity in clinical trials and genomic databases also drove the development of large, diverse patient cohorts, such as that developed by the Prostate Cancer Transatlantic Consortium and presented at the 15th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, held September 16-19, 2022, in Philadelphia.
Lastly, Chakravarty and colleagues expressed the importance of new biomarkers to better define patients who will benefit most from a given therapy. Protein markers of driver gene expression, rather than genomic or transcriptomic markers, were found to be especially important to predict the efficacy of ADCs, as membrane presentation of these proteins is crucial for ADC function. Chakravarty and colleagues emphasized the importance of evidence-based protein level cutoffs with well-validated assays to determine patient eligibility for such drugs.
The momentum from these new approvals, as well as the scientific advancements that made them possible, should spur the field toward additional discoveries and approvals in the coming year. We celebrate the progress made in 2022 and look forward to the breakthroughs that 2023 will bring.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











