Pliant Therapeutics Reports Positive Results in Bexotegrast Trial for Liver Fibrosis
05 February 2024 | Monday | News

Image Source : Public Domain
Bexotegrast (PLN-74809) at 320 mg was well tolerated over 12 weeks of treatment with no drug-related severe or serious adverse events; No safety concerns identified across all dose cohorts
Bexotegrast at 320 mg reduced liver fibrosis markers ELF and PRO-C3 and showed improvements in hepatocyte function and bile flow by contrast MRI imaging relative to placebo at Week 12
The 320 mg data continue to demonstrate antifibrotic effects of bexotegrast, consistent with previous findings
The trial’s exploratory efficacy endpoints assessed changes in the liver fibrosis markers, Enhanced Liver Fibrosis (ELF) score and PRO-C3 levels, as well as liver biochemistry and magnetic resonance imaging (MRI) of the liver. Consistent with the results from the lower doses tested, bexotegrast-treated patients at the 320 mg dose showed a reduction in both ELF score and PRO-C3 levels relative to placebo at Week 12. Bexotegrast-treated patients also showed stabilization of alkaline phosphatase (ALP) levels, relative to an increase on placebo at Week 12. In addition, MRI imaging continued to show evidence of improved hepatocyte function and bile flow with bexotegrast at the 320 mg dose relative to placebo.
INTEGRIS-PSC is a multinational, randomized, dose-ranging, double-blind, placebo-controlled Phase 2a trial evaluating bexotegrast at once-daily oral doses of 40 mg, 80 mg, 160 mg, 320 mg or placebo for 12 weeks in 121 patients with PSC. The 320 mg group enrolled 27 patients in the active arm and added 9 new patients to the pooled placebo arm. We believe INTEGRIS-PSC to be the first randomized clinical trial to use an enrichment strategy to enroll patients with suspected moderate to severe liver fibrosis based on liver stiffness measure, ELF score or historical liver biopsy. Baseline characteristics of the trial population reflected this enrichment. The 320 mg dose group will continue until all patients have been treated for at least 24 weeks, with final data expected in mid-2024.
“Results from INTEGRIS-PSC continue to build on the favorable safety and tolerability data for bexotegrast which is critically important in the setting of vulnerable patient populations and the need for chronic therapies,” said Éric Lefebvre, M.D., Chief Medical Officer of Pliant. “As the therapeutic profile of bexotegrast comes into focus with these data, it’s encouraging to see bexotegrast’s treatment effects manifested across multiple endpoints, suggesting its potential to impact PSC where therapies are urgently needed. I look forward to additional data from this trial in mid-2024 and upcoming discussions with regulatory authorities surrounding potential next steps.”
Bexotegrast 320 mg was Well Tolerated with No Drug-Related Severe or Serious Adverse Events
The primary endpoint of the INTEGRIS-PSC trial is the evaluation of the safety and tolerability of bexotegrast. The secondary endpoint is an assessment of its pharmacokinetics.
Bexotegrast at the 320 mg dose was well tolerated with no dose relationship observed for adverse events. Of the 27 patients treated with bexotegrast at the 320 mg dose, 26 (96%) completed 12 weeks of treatment with no drug-related severe or serious adverse events (SAE). Most treatment-emergent adverse events (TEAEs) were mild or moderate in severity and consistent with PSC disease symptoms. In addition, adverse events of pruritus and cholangitis occurred less frequently on all doses of bexotegrast relative to placebo. Patients in the trial who had concomitant inflammatory bowel disease (IBD) saw no change in their IBD symptoms as measured by partial Mayo Score while on treatment.
Bexotegrast total and unbound plasma concentrations increased with dose.
Bexotegrast 320 mg Demonstrated Antifibrotic Activity in a PSC Population with Suspected Moderate to Severe Liver Fibrosis at Week 12
The exploratory endpoints of the INTEGRIS-PSC trial include changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and MRI imaging.
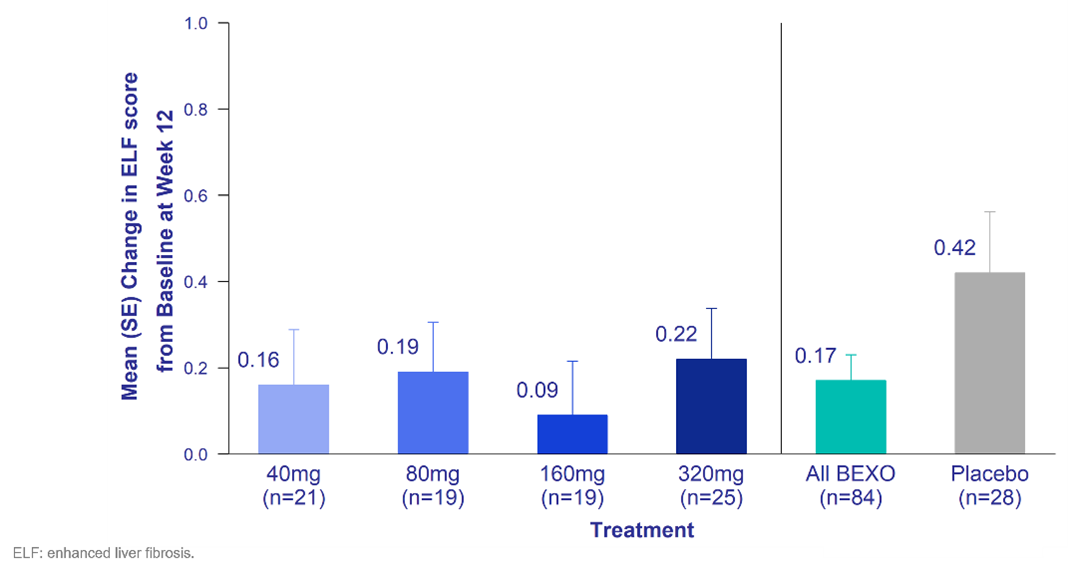
Figure 1. ELF Score – Change from Baseline at Week 12
Consistent with the lower doses tested, bexotegrast at 320 mg reduced ELF score relative to placebo at Week 12. The ELF score is a well-established prognostic marker of liver disease severity and liver-related events in patients with advanced fibrosis.1 ELF is strongly associated with transplant‐free survival in PSC and may be useful as a surrogate marker in clinical trials.2
Consistent with the lower doses tested, bexotegrast at 320 mg reduced PRO-C3 levels relative to placebo. PRO-C3 is a biomarker of active fibrogenesis with higher levels associated with greater disease activity.3
MRI relative enhancement using gadoxetate contrast is a measure of hepatocyte function, with increased enhancement suggesting improved hepatocyte function.4,5 Consistent with the lower doses tested, bexotegrast at the 320 mg dose showed an increase in relative enhancement on contrast MRI compared to a decrease observed in the placebo group at Week 12. In addition, consistent with the lower doses tested, bexotegrast at the 320 mg dose reduced time to arrival to the common bile duct compared to placebo, suggesting improved bile flow.6
Patients with PSC often experience pruritus, or itch, as part of their disease.7 Bexotegrast at the 320 mg dose demonstrated statistically significant reductions in the Itch Numerical Rating Scale relative to placebo at Week 12.
“Consistent with prior observations from the INTEGRIS-PSC trial, bexotegrast continues to demonstrate a very favorable safety profile while also maintaining efficacy signals,” said Kris V. Kowdley MD, AGAF, FAASLD, FACP, FACG, Director, Liver Institute Northwest and Professor of Medicine, Elson S. Floyd College of Medicine at Washington State University. “These promising results present a strong rationale for the further study of bexotegrast in patients with PSC as part of a larger late-stage trial.”
Bexotegrast in PSC Clinical Development Next Steps
The Company is planning to share these data from the INTEGRIS-PSC trial with regulatory authorities to discuss the potential path to registration.
We would like to thank our INTEGRIS-PSC investigators and their study teams for their dedication in support of the successful execution of this trial. Special thanks to the INTEGRIS-PSC clinical trial participants, their families and support networks for helping us advance this promising program.
Background on Primary Sclerosing Cholangitis
PSC is a rare, progressive liver disease of unknown origin, which frequently occurs in the setting of inflammatory bowel disease. PSC affects more than 30,000 patients in the United States and over 100,000 patients worldwide. The disease can occur in all ages, genders, and races. PSC is characterized by inflammation and fibrosis, with progressive liver and biliary damage leading to cirrhosis and liver failure. Currently there are no FDA or EMA-approved therapies for patients with PSC. Therefore, there is a high unmet need for new therapeutic options to address the symptoms and modify the disease progression of this grievous illness.
INTEGRIS-PSC Multinational Phase 2a Trial of Bexotegrast (NCT04480840)
INTEGRIS-PSC is a Phase 2a, multinational randomized, dose-ranging, double-blind, placebo-controlled trial evaluating the safety, tolerability, and pharmacokinetics of bexotegrast administered over 12 weeks in patients with IPF. Patients were enrolled in doses of 40 mg, 80 mg, 160 mg or 320 mg, with a 3:1 randomization ratio (active:placebo) and stratification based on use of ursodeoxycholic acid (UDCA). The primary endpoint is the evaluation of bexotegrast safety and tolerability and the secondary endpoint is the assessment of pharmacokinetics across the range of doses. Exploratory endpoints will measure changes in liver fibrosis markers, ELF and PRO-C3, liver biochemistry and liver imaging.
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks











