Fujitsu Launches Major Initiative to Attract Global Clinical Trials to Japan, Tackling the Nation's 'Drug Loss' Issue
26 August 2024 | Monday | News
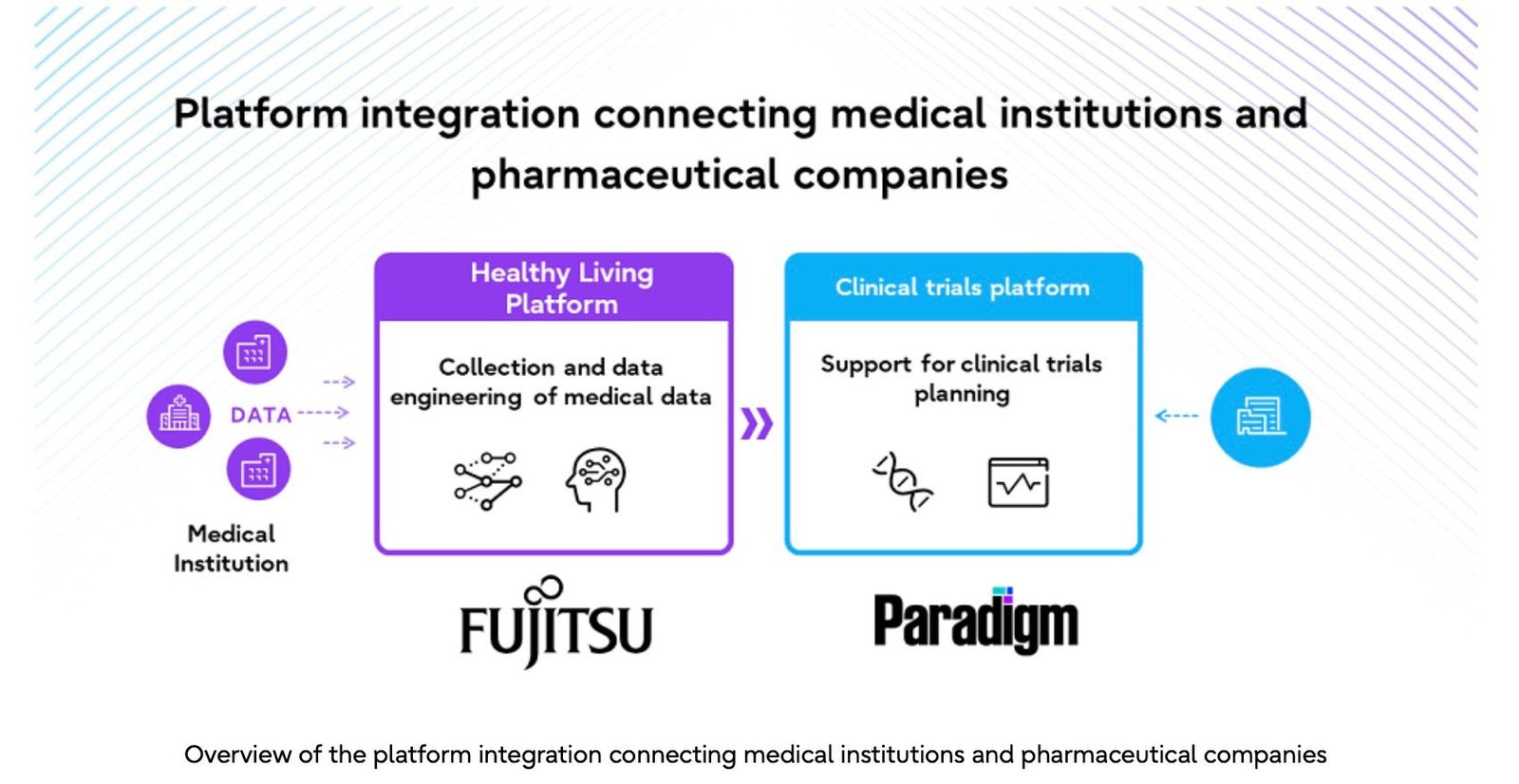
Fujitsu announced that it will begin initiatives to attract global clinical trials to Japan and tackle the ‘drug loss’ issue by working with pharmaceutical companies and medical institutions to build a new medical data ecosystem. The efforts come under the Fujitsu Uvance Healthy Living, which aims to improve people’s well-being.
In July 2024, Fujitsu formed a strategic partnership with Paradigm Health, Inc., a US startup that provides one of the world’s most advanced clinical trial platforms. Through utilizing this platform, Fujitsu’s Healthy Living Platform, and the Fujitsu Kozuchi AI service, Fujitsu and Paradigm will facilitate the collection and processing of data stored at medical institutions and speed up the clinical trial planning process.
In addition, starting today, Fujitsu will release Patient-centric Clinical Trials, a new service for automatically creating clinical trial documents using Fujitsu’s proprietary large language model (LLM). This service will be positioned in the company’s Healthy Living portfolio.
Fujitsu will continue to expand its support to include not only clinical trial planning, but also implementation, and will work to solve issues throughout the entire clinical trial process. Through these efforts, Fujitsu aims to secure 20.0 billion yen in revenue in fiscal 2030, as well as contribute to achieving a society in which patients can quickly obtain the medicine they need and choose the treatment that best suits them.
In Japan, patients who participate in clinical trials are dispersed across multiple hospitals making the process costly and time consuming. There has been an increasing number of cases in which Japan is excluded from regions targeted for global clinical trials. The impact of Japan’s drug pricing policy is also contributing to this situation.
As a result, the number of drugs that are in use outside of Japan but are not approved for use in Japan had risen to 143 as of March 2023 (source: the Japanese Ministry of Health, Labour and Welfare of Japan).
Overview of the Initiatives
1. Accelerating Digitization in the Area of Clinical Trials through Fujitsu’s Partnership with Paradigm
Fujitsu will work with Paradigm, a US startup that provides pharmaceutical companies and medical institutions with one-stop support for the clinical trial process, to develop a new clinical trial data ecosystem that utilizes medical data. Paradigm’s clinical trial platform, which hosts many global clinical trials and has a long track record of success, will be combined with Fujitsu’s operational support expertise and cutting-edge technologies in the area of healthcare and life sciences in Japan.
Clinical data, such as medical data and genomic data, will be collected from medical institutions through Fujitsu’s Healthy Living Platform. Fujitsu’s AI service, Fujitsu Kozuchi, will process this data so that it is compliant with the necessary laws and regulations, and it will be provided to Paradigm. In addition, Paradigm will use its clinical trial platform to analyze the data and provide pharmaceutical companies with the insight needed to plan and conduct clinical trials. This will allow pharmaceutical companies to better understand the situation at the medical institutions as well as the distribution of patients during the planning phase of the clinical trial, and significantly optimize the clinical trial design process.
Medical institutions will also be able to quickly access information about clinical trials in which patients are able to participate through Paradigm’s clinical trial platform, making it easier for medical institutions to encourage patients to participate in clinical trials at the appropriate time.
Fujitsu will launch these solutions starting in September 2024, beginning with providing support to core clinical research hospitals.
2. Providing Patient-centric Clinical Trials, a New Offering for Optimizing Clinical Trial Workflows through a Specialized LLM
In Japan, the hundreds of clinical trial-related documents that pharmaceutical companies need to produce to develop a new drug are still being created and managed manually. However, by using its LLM specialized for clinical trials, Fujitsu will offer a service that can automatically create these documents and ensure that they are in compliance with regulations. This service will be provided in Japan as the Patient-centric Clinical Trials offering as part of its Healthy Living portfolio.
In conducting a PoC of this offering with a pharmaceutical company, the LLM was able to automatically create approximately 80% of each document. According to Fujitsu’s estimates, this is expected to lead to a reduction in the total time required to create documentation of up to 50%. Through this offering, Fujitsu aims to make a significant contribution to optimizing the clinical trial planning and implementation workflow.
Future Plans
Fujitsu will work to accelerate the digitalization of the clinical trial environment in Japan by providing total support for the entire clinical trial process, including planning and implementation. Through these efforts, Fujitsu aims to create the groundwork for increasing the number of global clinical trials conducted in Japan, solve the issue of drug loss and contribute to achieving a society in which all individuals can choose to live their lives in the way that suits them.
Statement from Kent Thoelke, CEO of Paradigm Health, Inc.
Paradigm is excited to be partnering with Fujitsu to maximize clinical trial efficiency in Japan. This collaboration will modernize the clinical trial model and provide unprecedented access to clinical trials for patients across Japan. The partnership will leverage Paradigm’s industry leading Platform and Fujitsu’s expansive healthcare solutions to increase clinical trial patient accrual, decrease overall drug development timelines and costs, while working to eliminate drug loss in Japan. We believe that this collaboration will establish Japan as an essential part of the global clinical trials and drug development ecosystem while ensuring that Japanese patients have equitable access to the best possible care options.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











