Arctic Vision Announces First Patient Enrolled in Phase III Clinical Trial of ARVN003 for Presbyopia
05 July 2022 | Tuesday | News
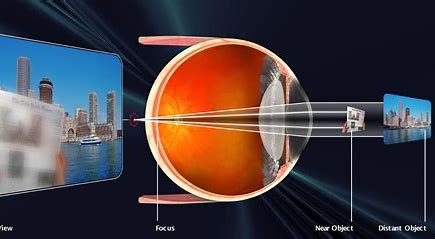
Image Source : Public Domain
Arctic Vision, a China-based biotech company focused on innovative ophthalmic therapies, today announced that the first patient has been enrolled in a Phase III clinical study evaluating ARVN003, a proprietary pilocarpine formulation leveraging its micro dosing platform Optejet®, as a treatment to temporarily improve vision in adults with presbyopia in China.
The Phase III study is double-masked, placebo-controlled, randomized, and multicenter trial evaluating the efficacy and safety of ARVN003 in achieving temporary improvement of vision in adults with presbyopia. It is the first clinical trial approved in China for presbyopia drugs and Arctic Vision's study marks the first patient enrollment in a Phase III clinical trial for presbyopia drugs in China.
Presbyopia is a physiological condition that makes it difficult to read and work in near distance. It is caused by the hardening of the lens and weakening of the ciliary muscle and often occurs with aging. Pre-presbyopia typically affects people between the ages of 35 and 45; early presbyopia affects people between the ages of 45 and 52; and late presbyopia, also known as absolute presbyopia, affects people over the age of 52. Today, nearly a quarter of the world's population is affected by presbyopia. With China's rapidly ageing population, the nation is seeing a year-on-year increase in the number of people suffering from presbyopia. Latest data revealed more than 390 million people in China are diagnosed with presbyopia in 2021.
Current treatment options for presbyopia include presbyopic reading glasses, contact lenses and surgery. However, due to limited treatment options and inadequate scientific understanding of the disease, many presbyopia patients in China do not receive intervention or vision correction in time. Untreated and escalated, presbyopia adversely impacts vision, quality of work and life, and the psychological well-being of middle-aged and elderly people. It might also increase financial burden on families and society.
Professor Jia Qu, the Principal Investigator and renowned leader in China's ophthalmology sector – President of Eye Hospital of Wenzhou Medical University said, "With China's rapidly aging population and a growing number of younger presbyopia patients, there is exponential demand for effective, safe, and convenient presbyopia treatments. We are excited to lead China's first clinical study of presbyopia medication and look forward to the approval of ARVN003 in the near future."
Dr. Qing Liu, Co-Founder and Chief Medical Officer at Arctic Vision added, "ARVN003's significant clinical progress is encouraging. This clinical achievement follows the successful dosing of the first DME patient in Asia with 锋脉® (Feng Mai) or Arcatus™ (ARVN001), and is testimony of the strength of Arctic Vision's proprietary microdose array print (MAP™) technology in the public eye health sector. We strive to make ARVN003 the first approved presbyopia drug in China, and benefit more presbyopia patients with innovative and diverse treatment options that will help them see and live better."
Arctic Vision obtained an exclusive license in August 2020 for the development and commercialization of ARVN003 (MicroLine) in Greater China and South Korea from Eyenovia, a U.S.-based clinical-stage biopharma company. In May 2021, Eyenovia announced positive results from the first Phase 3 MicroLine study, VISION-1, in the U.S. In that trial, the primary endpoint was achieved with MicroLine 2% statistically superior to placebo, determined by improvement in high contrast binocular distance corrected near visual acuity measured in low light conditions two hours after treatment.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











