LumiraDx Receives WHO Emergency Use Listing for SARS-CoV-2 Ag Test
23 May 2022 | Monday | News
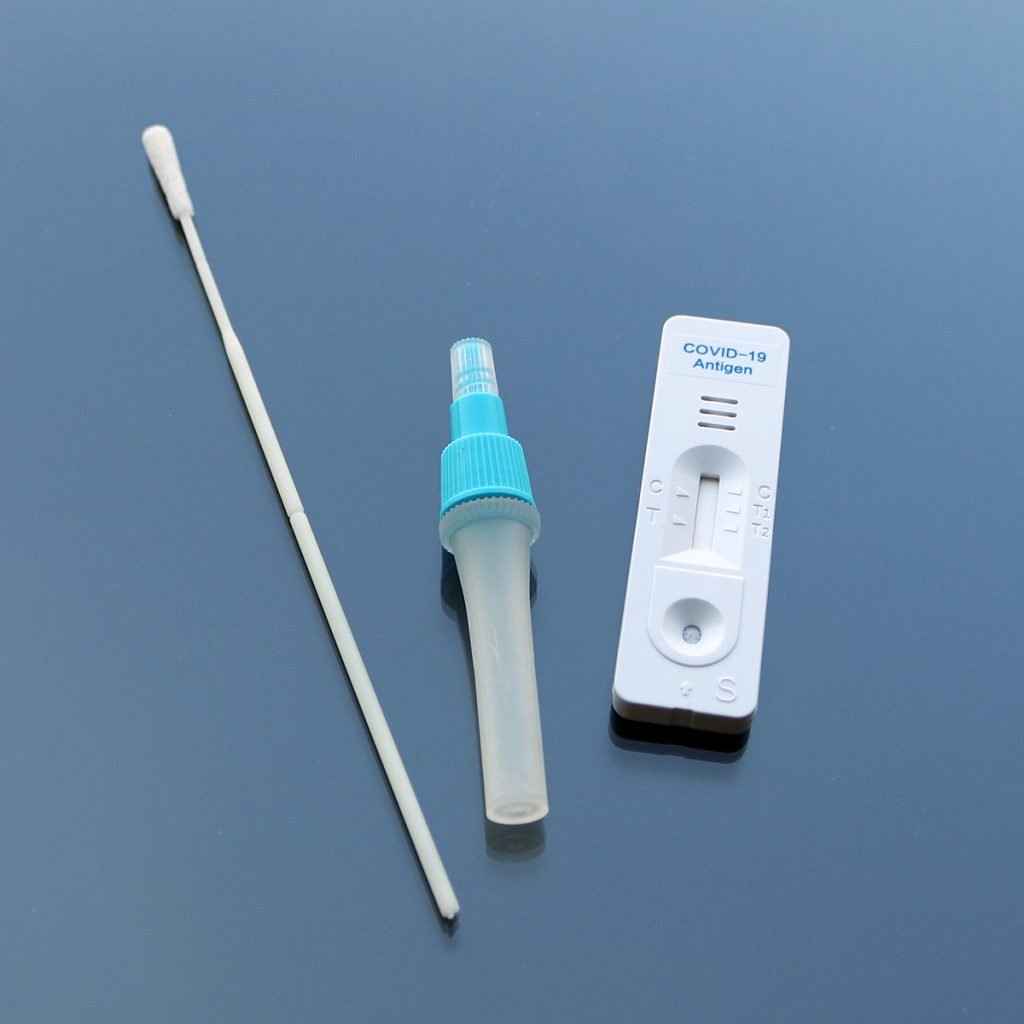
Image Source : Public Domain
The LumiraDx antigen test is a microfluidic immunofluorescence assay that delivers results for the direct and qualitative detection of nucleocapsid protein antigen in nasal swab specimens collected from the patient. Used with the LumiraDx multi-assay Platform, the test delivers rapid results at the point of care enabling physicians to immediately commence appropriate treatment and patient management to maximize health outcomes and minimize further spread of infection.
"The ongoing complexities of the evolving pandemic require multiple lines of defense," said Dr Nigel Lindner, Chief innovation Officer. "This important milestone lets us offer a reliable and affordable, high-performance testing alternative to better manage the pandemic burden and help reduce the strain on health systems worldwide."
Through a partnership with the Bill and Melinda Gates Foundation, LumiraDx has already distributed 5,000 Platforms in 49 African countries in a variety of care settings, including field clinics, airports, primary healthcare facilities, occupational health settings, and walk-through clinics. In addition to its SARS-CoV-2 Ag test, other assays in LumiraDx's pipeline for TB, diabetes, HIV-AIDS and other health conditions have the potential to make a meaningful impact on global health.
The decision by the WHO to include the LumiraDx SARS-CoV-2 antigen test in its EUL was based on an essential set of available quality, safety, and efficacy and performance data. In clinical studies the test demonstrated 95% positive agreement versus RT-PCR in patients tested within 12 days of the onset of symptoms. The LumiraDx antigen test joins a select group of antigen tests the WHO has included in its Emergency Use Listing and represents the first microfluidic assay to meet the criteria.
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











