Medtech Medical Devices
Lunit's Groundbreaking AI Research Transforms Medical Pathology with Computer Vision, Presented at CVPR 2023
Lunit (KRX:328130.KQ), a leading global provider of AI-powered cancer diagnostic solutions, announced its participation in the Conference on Computer...
June 13, 2023 | News
Translumina bolsters its German presence with acquisition of Lamed GmbH
Established in 1989, Lamed is a leading market access platform of vascular and cardiovascular products. It covers 1,000+ hospitals through its GPO network ...
June 07, 2023 | News
Seegene Obtains IVDR Certification for 30 Diagnostic Assays
Seegene certifies diagnostic assays for digestive, respiratory, women's diseases, and human papillomavirus to meet IVDR requirements New regulation...
June 02, 2023 | News
Lunit and the JNCCHE Partner in AI Pathology to Advance Precision Oncology
Lunit (KRX:328130.KQ), a global provider of AI-powered cancer diagnostic solutions, today announced collaboration with the National Cancer Center...
May 31, 2023 | News
Tianlong Assists Thailand Hospitals in Early Hepatitis Detection with Molecular Diagnostics
Globally, more than 350 million people are living with viral hepatitis, and 9 in ten people living with hepatitis are unaware of their diagnosis. Tianlong'...
May 26, 2023 | News
Lunit's AI-Biomarker Platform Shows Predictive Value at ASCO 2023
Lunit (KRX:328130.KQ), a global leader of AI-powered cancer diagnostics and therapeutics solutions, is set to make a significant impact at the American Soc...
May 26, 2023 | News
Abbott's Dual-Chamber Leadless Pacemaker Study Meets Primary Endpoints
Abbott's AVEIR™ DR i2i™ IDE study is the industry's first prospective study on the safety and performance of the world's first dual-chamber l...
May 22, 2023 | News
Medtronic Extravascular ICD Trial Yields Positive Safety and Effectiveness Results
Late-breaking data at Heart Rhythm 2023 underscore performance of first-of-its-kind investigational defibrillator with the lead placed under the breas...
May 22, 2023 | News
International Healthcare Week returns to showcase Hong Kong's strengths in healthcare innovation and investment
The second International Healthcare Week (IHW), organised by the Hong Kong Trade Development Council (HKTDC) and supported by a wide range of healthcare se...
May 04, 2023 | News
Olympus Announces FDA Clearance of New Endoscopy System and Compatible Endoscopes
Olympus, a global technology leader in designing and delivering innovative solutions for medical and surg...
May 04, 2023 | News
Medtronic receives FDA approval for its next generation Micra leadless pacing systems
Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced it has received U.S. Food and Drug Administration (FDA) approval ...
May 02, 2023 | News
Fapon Biotech Announces Chemiluminescence One-Stop Solution Strategy in India at Medical Fair India 2023
Fapon chemiluminescence one-stop solution is designed to provide a complete range of chemiluminescence products and services, consisting of different level...
April 27, 2023 | News
500 women join Australia's landmark maternity medical device study
The study was led by Principal Investigators Professor Michael Nicholls, Professor Andrew Bisits and Michelle de Vroom at two Aust...
April 20, 2023 | News
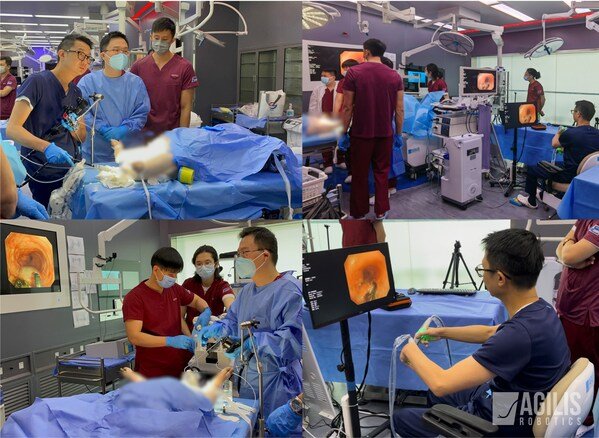
Agilis Robotics tests animal trial for GI indication, aims 510(k) clearance by 2025 for improved endoscopic surgery.
The system by Agilis Robotics offers a novel combination of features that set it apart from conventional surgical robotic systems. With a remarkably compac...
April 19, 2023 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News