Med’s Industry
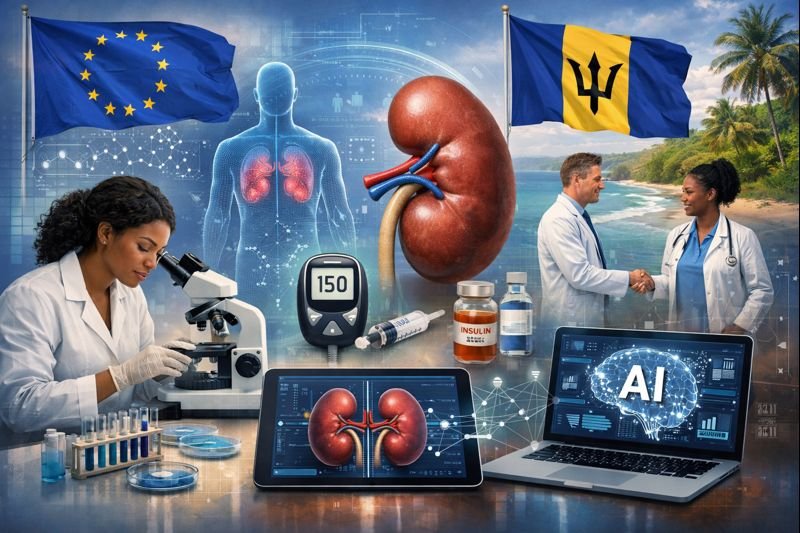
BioMed X Launches EU Backed AI Precision Medicine Project With Barbados To Address Early Diabetic Kidney Disease
The new project, supported by the European Union’s PharmaNext Programme, focuses on population-specific molecular profiling and AI-driven precision m...
January 28, 2026 | News
Merz Therapeutics Submits XEOMIN For Paediatric Spasticity Indication Across Europe
Merz Therapeutics, a leading player in neurology-focused specialty pharma, announced that it has completed the regulatory submission for XEOMIN®&...
January 27, 2026 | News
Imagine Devices Inc. Partners With Neotech Products To Advance Trinity Tube For Neonatal Critical Care
Imagine Devices Inc., a medical device company focused on advancing neonatal and pediatric critical care, announced a strategic partnership with Neotech Pr...
January 27, 2026 | News
Mindray Medical Sets Decade Long Growth Agenda At J.P. Morgan Healthcare Conference Built On Globalisation Recurring Revenue And Intelli Digitalisation
May Li, Board Secretary of Mindray Medical (300760.SZ), delivered a keynote speech at the 44th J.P. Morgan Healthcare Conference (JPM Conference) on Januar...
January 26, 2026 | News
Gene Solutions And Pangea Laboratory Partner To Advance US Clinical Validation Of Liquid Biopsy Tests
Insilico Medicine and Hygtia Therapeutics, an incubatee of Shenzhen Pengfu Fund of Fosun Health Capital and Fosun Pharma, have entered into an exclusive ...
January 22, 2026 | News
Zylox Tonbridge Accelerates Global Expansion With Strategic Acquisition Of Optimed
Marks a major milestone in Zylox-Tonbridge's long-term commitment to global expansion Builds an integrated global commercialization platform to accel...
January 19, 2026 | News
Thermo Fisher Scientific Introduces Invitrogen TaqMan Cells To CT HepatoExpress Kit For Faster Liver Based Gene Expression Analysis
Thermo Fisher Scientific has launched the Invitrogen TaqMan Cells to CT HepatoExpress Kit, a new solution designed to simplify and accele...
January 16, 2026 | News
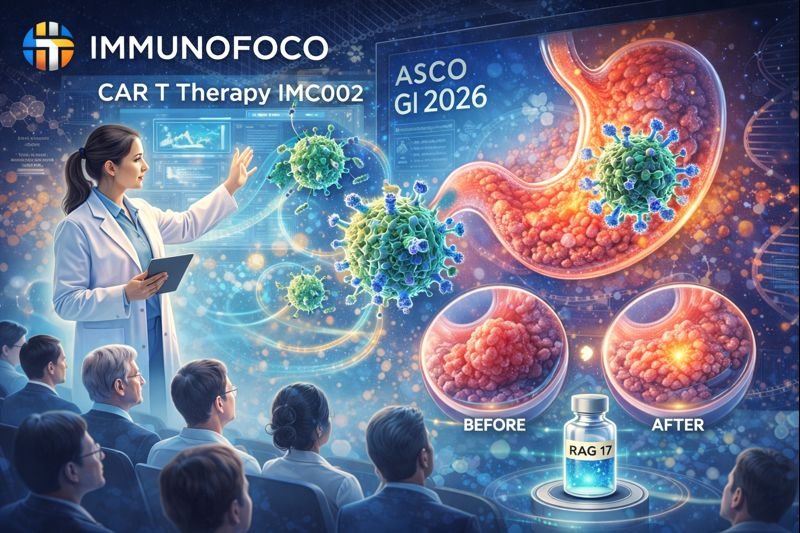
Immunofoco CAR T Therapy IMC002 Posts Strong Responses In Advanced Gastric And Gej Cancers At Asco Gi 2026
Immunofoco, a clinical-stage biotechnology company advancing innovative CAR-T cell therapies for solid tumors, recently announced clinical data from ...
January 15, 2026 | News
Ractigen Doses First Patient In Phase Two Trial Of RAG 17 For SOD1 Mutated Als
The initial dosing occurred at Second Affiliated Hospital, Zhejiang University School of Medicine, under the leadership of Dr. Zhi-Ying Wu, head...
January 15, 2026 | News
Allele Joins Arpa H Programme To Bioprint Patient Specific Livers With Uc San Diego
Allele Biotechnology & Pharmaceuticals, Inc. (Allele), a San Diego-based technology development and cell manufacturing company, announced its par...
January 15, 2026 | News
Alamar Launches NULISAqpcr AD 5 Plex Blood Test To Advance Early Alzheimer Detection
Alamar Biosciences, Inc. ("Alamar"), a leader in precision proteomics dedicated to advancing the early detection of disease, is proud to announce the launc...
January 14, 2026 | News
Ventaris Surgical Raises $30 Million Series A To Transform Kidney Stone Surgery
Ventaris Surgical, Inc., a San Carlos, CA-based medical device company developing a next-generation system for kidney stone treatment, announced the ...
January 12, 2026 | News
AirNexis Secures Global Rights To Phase 2 COPD Asset AN01 In $200 Million Series A Backed By Frazier And OrbiMed
AirNexis acquires exclusive rights to develop AN01 outside of China from Haisco Pharmaceutical Group Company founded by Frazier Life Sciences, which led...
January 12, 2026 | News
One Genomics And CiRA Foundation Forge AI Powered Genome Engineering Alliance To Accelerate De Aging Science
AI-Focused Genomics Company Advancing De-Aging Science Collaborates with Leading iPS Cell Manufacturing and Research Foundation One Genomics, an...
January 12, 2026 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News