LifeScience Vaccines
Novotech Client SK bioscience Achieves SKYCovione(TM) COVID-19 Vaccine Approval in Korea
SK bioscience and GSK recently announced the successful authorization:SK bioscience announced that 'SKYCovione(TM),' South Korea's first COVID-19 vaccine c...
July 14, 2022 | News
U.S. Government Secures 3.2 Million Doses of Novavax COVID-19 Vaccine
Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious dise...
July 12, 2022 | News
GSK Spreads Awareness on Vaccine-Preventable Diseases, Attempting to Bridge the Vaccination Gap in Malaysia
GSK is a science-led global healthcare company, delivering vaccines that help protect people at all stages of life. GSK's new purpose is to unite science, ...
July 01, 2022 | News
Clinical Trial Approval For mRNA Covid-19 Vaccine For Omicron Variant In The Philippines
R520A is an mRNA COVID-19 vaccine specifically targeting the Omicron variant developed by Wuhan Recogen Biotechnology Co., Ltd., a subsidiary of the Compan...
June 28, 2022 | News
Nippon Express Launches New Transport Service Using Environment-friendly Isothermal Packaging
Nippon Express Co., Ltd., a group company of Nippon Express Holdings, Inc., has become the first Japanese logistics company to collaborate with EMBAL...
June 22, 2022 | News
EpiVax Poxvirus Vaccine Candidate Predicted to be Effective Against Monkeypox
The development of this vaccine was funded by the National Institutes of Health (SBIR grant #R43AI058376). The company is looking for a strategic part...
June 13, 2022 | News
FDA Recommends EUA of Novavax COVID-19 Vaccine for People Aged 18 Years and Older
If Emergency Use Authorization is granted by the FDA, the Novavax COVID-19 vaccine would become the first protein-based COVID-19 vaccine available in the...
June 09, 2022 | News
ProteoGenix, Aseem Healthcare, and Trident Biopharm Solutions announce a new SARS-CoV-2 antibody cocktail
ProteoGenix, Aseem Healthcare, and Trident Biopharm Solutions Announce a New Antibody Cocktail Effective Against Major Variants of SARS-CoV-2 Prot...
June 08, 2022 | News
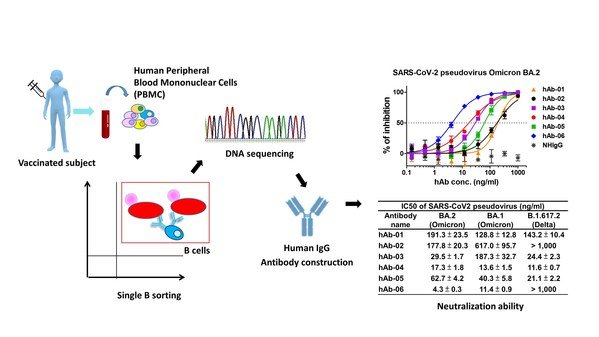
Taiwan's AcadeMab develops groundbreaking therapy for COVID-19 Omicron variant with potent neutralizing efficacy
AcadeMab's treatment specifically targets the SARS-CoV-2 Omicron variant – currently the most common strain of COVID infections around the world. "I...
May 30, 2022 | News
CanSinoBIO's COVID-19 Vaccine Convidecia™ Receives WHO Emergency Use Listing
In addition to shortening the vaccination cycle by leveraging the advantages of its single-dose regimen, Convidecia™ can be stably transported and st...
May 20, 2022 | News
ASEAN to build its own CDC to mitigate another pandemic risk
Indonesian Health Minister Budi Gunadi Sadikin said that Southeast Asia's regional bloc agreed to solidify the three main pillars of pa...
May 18, 2022 | News
Clinical Trial Approval For ReCOV In The PRC And The Overseas Clinical Trial Progress Of ReCOV
As disclosed in the prospectus of the Company dated March 21, 2022, the Group had initiated subject enrollment for the phase II/III clinical trial for...
May 06, 2022 | News
Promising COVID oral antiviral being co-researched by Healion Bio and FUJIFILM Toyama
Dr. Sina Bavari, co-founder of Healion Bio, noted "Highly contagious pathogens can rarely be stopped by testing, vaccines, and hospital therapeutics alone....
April 30, 2022 | News
Lessons Learned from COVID-19 Vaccine Trials
IQVIA Institute releases report on Lessons Learned from COVID-19 Vaccine Trials Solutions for you to dri...
April 28, 2022 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News