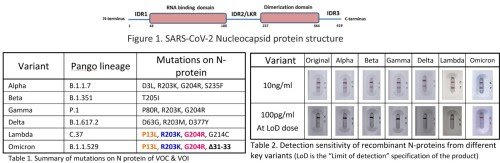
LAB Testing
Singapore Grants Approval for INDICAID COVID-19 Rapid Antigen Test
PHASE Scientific International LTD (PHASE Scientific), today announced that its INDICAID™ COVID-19 Rapid Antigen Test (INDICAID&...
January 27, 2022 | News
Eppendorf expands its portfolio to address customers’ separation needs
The introduction of the portfolio under the Eppendorf umbrella began with the launch of the CP22N and CP30NX high-speed, floor-standing centrifuges in 2021...
January 26, 2022 | News
Seegene to Launch New COVID-19 PCR Test for Mass Testing
Seegene Inc. (KQ 096530), South Korea's leading molecular diagnostics company, announced today the upcoming launch of the Allplex™ SARS-CoV...
January 20, 2022 | News
Seegene to export COVID-19 test that detects COVID-19 and Omicron
Seegene Inc. (KQ 096530), South Korea's leading molecular diagnostics company, announced today that it has delivered over five million COVID...
January 18, 2022 | News
LumiraDx Lab Analysis Confirms its COVID-19 Antigen Test Detects the Omicron Variant
LumiraDx (Nasdaq: LMDX), a next-generation point of care diagnostics company, today announced that results from ongoing testing and monitoring of COVID-19 ...
January 17, 2022 | News
Singapore biotech firm JN Medsys develops a PCR test kit
Singapore-based biotech firm, JN Medsys announces that it has developed a new PCR kit that detects for SARS-CoV-2 and identifies if the Omicron variant is ...
January 13, 2022 | News
LumiraDx receives CE marking after successful CRP test
Quantitative results within 4 minutes of sampling facilitate rapid clinical decision-making at the point of care (POC) LumiraDx (Nasdaq: LMDX),...
January 12, 2022 | News
Siemens Healthineers Announces FDA EUA For CLINITEST® Rapid COVID-19 Antigen Self-Test
Rapid antigen testing provides results in just 15 minutes and the information can help reduce the risk of COVID-19 exposure. Through nationwide retail d...
December 30, 2021 | News
Labcorp Strengthens Broader Access to Innovative Diagnostics Improves Patient Outcomes and Accelerates Clinical Trials
Labcorp (NYSE: LH), a leading global life sciences company, today announced that it has entered into a definitive agreement to acquire Personal Genome...
December 27, 2021 | News
Zynex Announces Acquisition of Kestrel Labs
Zynex, Inc. (NASDAQ: ZYXI), an innovative medical technology company specializing in the manufacture and sale of noninvasive medical devices for...
December 24, 2021 | News
How to Choose the Right At-Home COVID Test
Insight follows announcement that the Biden administration will purchase 500 million rapid COVID-19 tests and distribute free of cost to Americans With th...
December 22, 2021 | News
MOH Singapore granted Acumen Diagnostics COVID-19 offsite PCR testing license
PCR testing services will be rolled out to an initial 10 clinics by 31 December 2021- Vaccinations and a robust testing regime remain the best strate...
December 16, 2021 | News
ARISTA Biotech Confirms Antigen Test Effectiveness Against Omicron and other COVID variants
On 26th November 2021, the World Health Organization (WHO) announced and designated variant B.1.1529 named Omicron, a newly identified variant first identi...
December 15, 2021 | News
Seegene Rapidly Introduces New PCR Test that Identifies the Omicron Variant
South Korea’s leading molecular diagnostics company, today unveiled another series of diagnostic test that detects the ever-changing COVID-19 variant...
December 13, 2021 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News