Countries North America
Otsuka Advances Centanafadine With FDA Filing To Bring a First in Class ADHD Therapy to Children, Adolescents, and Adults
Centanafadine is an investigational compound for the treatment of ADHD in children, adolescents, and adults. It is a first-in-class norepinephrine, dop...
November 26, 2025 | News
BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
BioMed X, a leading external innovation hub for pharma, announced the launch of a new collaborative research project with AbbVie, hosted at the BioMed...
November 26, 2025 | News
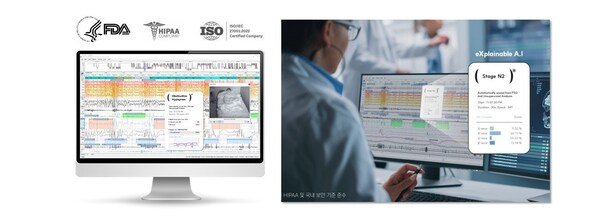
HoneyNaps Drives Faster Clinical Trials with FDA Cleared AI Sleep Analysis Platform
Enhancing Clinical Efficiency with AI Sleep Analysis: Cutting Both Cost and Time Joint Clinical Studies with CROs: SOMNUM™ AI Sleep Scoring D...
November 25, 2025 | News
AGC Biologics and AAVantgarde Forge New Partnership to Advance Dual AAV Gene Therapies for Inherited Blindness
Partnership accelerates AGC Biologics' leadership in the AAV market, applying its BravoAAV™ platform to complex gene therapies for inherited blindn...
November 24, 2025 | News
Pfizer and Astellas Gain Landmark FDA Approval for PADCEV and Keytruda in Cisplatin Ineligible Muscle Invasive Bladder Cancer
PADCEV plus Keytruda is the first and only approved perioperative treatment regimen that can significantly improve survival over current standard of ca...
November 24, 2025 | News
Everest Medicines Begins Global Phase I Trial of EVM14 as First Patient Receives Dose in the United States
The first patient had received the first dose of EVM14 injection in a global, multi-center Phase I clinical trial of Everest Medicines' Tumor-Associated ...
November 24, 2025 | News
Aspen Neuroscience Secures $115 Million Series C to Advance Personalised Cell Therapy for Parkinson’s Disease
Aspen Neuroscience, Inc., a clinical-stage biotechnology company pioneering autologous regenerative therapies, announced the closing of a $115 millio...
November 21, 2025 | News
Abbott to Acquire Exact Sciences for $21 Billion to Transform Cancer Diagnostics
Acquisition adds a new growth vertical to Abbott's already high single-digit growth profile, gaining leadership in the fast-growing $60 billion U.S. canc...
November 21, 2025 | News
Accord BioPharma Gains FDA Approval for Two Denosumab Biosimilars to Expand Access to Osteoporosis and Cancer Related Bone Care
Products will treat osteoporosis and skeletal-related events from certain types of bone cancer, expanding the company's biosimilar portfolio Accord BioPha...
November 21, 2025 | News
Hoth Therapeutics Joins NVIDIA Connect Program to Advance AI Driven Drug Discovery
Hoth Therapeutics, Inc. (NASDAQ: HOTH), a clinical-stage biopharmaceutical company focused on developing breakthrough therapeutics announced that it h...
November 21, 2025 | News
ReviR Therapeutics Wins CIRM Grant to Advance First in Class Dual Target Therapy for Huntington’s Disease
ReviR Therapeutics, a biotech company focused on developing innovative treatments for neurogenetic diseases, announced that it has been awarded a $4....
November 21, 2025 | News
Thermo Fisher FDA Clearance Expands Precision Pathway for HER2 Driven Lung Cancer Treatment
Test will serve as a companion diagnostic for Bayer’s HYRNUO™ (sevabertinib) to identify patients with NSCLC eligible for HER2-targeted therapy...
November 21, 2025 | News
CRScube Acquires Mednet to Build a Unified Global eClinical Platform Across Asia and North America
CRScube has announced the successful acquisition of Mednet, marking a key milestone in its global strategic growth and expansion into the North Ameri...
November 20, 2025 | News
Nona Biosciences Enters License Agreement with Pfizer to Advance Global Antibody Discovery
Nona Biosciences, a global biotechnology company providing integrated solutions for biological drug discovery and development from I to I® (Idea t...
November 20, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News