Countries Europe
ProBioGen to Operate GMP Manufacturing Hub at Berlin’s New Gene and Cell Therapy Centre
ProBioGen and the Berlin Institute of Health at Charité (BIH) announced that ProBioGen has been selected to operate the process...
September 17, 2025 | News
Thermo Fisher Scientific Introduces Gibco™ Efficient-Pro™ Medium (+) Insulin to Boost Titers by Up to 61% and Streamline Bioproduction
Next-generation medium delivers up to 61% higher titers1, faster workflows, and greater flexibility for insulin-dependent CHO cell lines Thermo Fisher Sci...
September 16, 2025 | News
Pulnovo Medical Secures Dual FDA IDE Approvals and CMS Coverage for PADN Clinical Trials in the U.S.
Pulnovo Medical, a globally recognized leader in medical devices for pulmonary hypertension (PH) and heart failure (HF), is proud to announce that its PADN...
September 15, 2025 | News
Lunit Secures Landmark Framework Agreement to Deploy Breast Imaging AI Across 1,500+ French Public Hospitals
Framework agreement with UniHA enables access to Lunit's breast AI solutions across 1,500+ French public hospitals, in collaboration with Fujifilm L...
September 12, 2025 | News
PlasmidFactory’s Dr Martin Schleef Receives Germany’s Coveted NRW Innovation Prize
The founder and long-standing CEO of PlasmidFactory GmbH, Dr. Martin Schleef, was honored in Düsseldorf with the Innovation Award 2025 of the St...
September 11, 2025 | News
AGC Biologics Seattle Site Achieves New Heights in Manufacturing Excellence
Manufacturing excellence positions Seattle site as premier destination for mammalian and microbial biologic production in thriving Pacific Northwest life...
September 11, 2025 | News
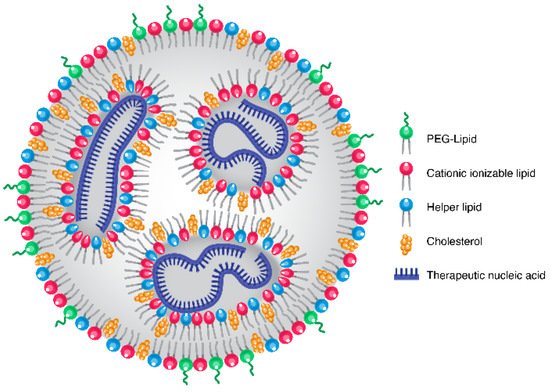
Evonik and Ethris Forge Strategic Alliance to Advance Next-Generation Lipid Nanoparticle Delivery
Evonik, a global specialty chemicals company, and Ethris, a clinical-stage biotechnology company pioneering next-generation RNA therapeutics and vaccines, ...
September 10, 2025 | News
Servier Acquires Kaerus Bioscience’s KER-0193 in Deal Worth Up to $450M
Servier, an independent international pharmaceutical group governed by a foundation, announced that it has entered into a definitive agreement with Kaerus ...
September 09, 2025 | News
Top 25 Biotech & Biopharma Leaders in Sustainable Innovation, 2025
In 2025, sustainable innovation has become a core focus in biotechnology and biopharmaceutical industries worldwide. Private companies are at the forefront...
September 08, 2025 | Analysis
China’s Biocytogen and Germany’s Merck KGaA Join Forces on Antibody-LNP Delivery Innovation
-Biocytogen Pharmaceuticals , a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative te...
September 05, 2025 | News
Thermo Fisher Scientific Launches Invitrogen™ UltraComp eBeads™ to Advance Accuracy in Spectral and Conventional Flow Cytometry
hermo Fisher Scientific announced the launch of Invitrogen™ UltraComp eBeads™ Spectral Unmixing Beads, a breakthrough solution designed t...
September 05, 2025 | News
Acer Expands SpatialLabs Pro With NVIDIA Omniverse, Vision Engineering, and Smart Surgery Integrations
Integration with NVIDIA Omniverse libraries via OpenXR runtime supports enhanced spatial awareness for industrial designs and simulations Partnership wi...
September 04, 2025 | News
Speech Processing Solutions Partners With Corti to Add Ambient AI Documentation to Philips SpeechLive
Speech Processing Solutions (SPS), the global leader behind Philips SpeechLive, announced a strategic partnership with Corti, the specialized AI infr...
September 04, 2025 | News
Lunit Secures Milestone European Deployment in Spain’s Valencian Community for AI-Powered Breast Cancer Screening
Milestone European deployment underscores growing global trust in Lunit's AI for population-wide breast cancer screening Lunit , a leading provider o...
September 03, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News