Countries China
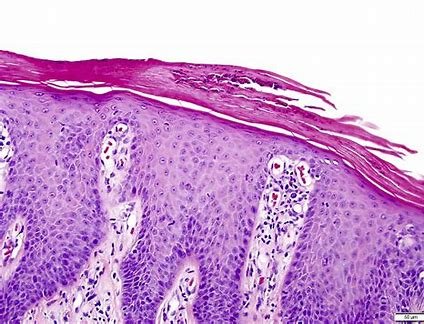
China Grants Full Approval to Everest Medicines’ NEFECON® as First and Only Etiological Treatment for IgA Nephropathy
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing, a...
May 07, 2025 | Regulatory
Beijing Tide Begins Phase II Trial of First-in-Class Non-Opioid CPSP Drug TRD205
Beijing Tide Pharmaceutical Co., Ltd., a subsidiary of Sino Biopharmaceutical Limited (1177.HK), has achieved a milestone in developing its first-in-class ...
May 06, 2025 | News
Fangzhou Launches Otsuka’s Iclusig® Online for Leukemia Care in China
Fangzhou Inc. , a leader in Internet healthcare solutions, announced the availability of Otsuka Pharmaceutical's third-generation tyrosine kinase inhi...
May 06, 2025 | News
Nutshell Therapeutics Receives FDA IND Clearance for First-in-Human Trial of NTS071 Targeting p53 Y220C Mutation
Nutshell Therapeutics ( Shanghai ) Co., LTD. received IND clearance from the FDA to initiate Phase 1 clinical ...
May 05, 2025 | News
InnoCare’s Zurletrectinib Granted Priority Review in China for NTRK Fusion-Positive Solid Tumors
InnoCare Pharma (HKEX: 09969), a leading biopharmaceutical company for the treatment of cancer and autoimmune diseases, announced that its new genera...
May 05, 2025 | News
JediCare, West China Hospital Complete First Human Study of Percutaneous Nodule Resection Device
JediCare Medical Technology Co., Ltd. a Shanghai, China based company ("the Company"), jointly with West China Hospital of Sichuan Univ...
May 02, 2025 | News
Innovent Biologics Doses First Patient in Global Phase 1 Trial of IBI3020, a First-in-Class Dual-Payload CEACAM5 ADC
Innovent Biologics, a world-class biopharmaceutical company that develops, manufactures, and commercializes high-quality medicines for the treatment ...
April 30, 2025 | News
Keymed Biosciences Advances CDH17-Targeted ADC CM518D1 Into Phase I/II Trials for Solid Tumors in China
Keymed Biosciences Inc. (HKEX: 02162) ("Keymed" or the "Company") today announced CM518D1, a CDH17-targeted antibody-drug conjugate (ADC) develop...
April 28, 2025 | News
Innovent Biologics Secures NMPA Approval for Limertinib as First-Line Therapy for EGFR-Mutated NSCLC in China
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality me...
April 28, 2025 | News
Doer Bio Begins Phase 2 Trial of Tri-Agonist DR10624 for MASLD and MASH Treatment
Zhejiang Doer Biologics Co., Ltd. ("Doer Bio"), a clinical stage biopharmaceutical company developing innovative biotherapeutics for metabolic diseases and...
April 24, 2025 | News
Sichuan Kelun-Biotech Secures FDA IND Clearance for First-in-Class ADC SKB518
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the "Company") announced that the Company has been granted the clearance of investigational new dru...
April 22, 2025 | News
Rona Therapeutics Secures IND Approval in China for siRNA-Based Hypertension Therapy RN1871
Rona Therapeutics, a biotechnology company pioneering innovative RNA-targeted therapies, announced that the Investigational New Drug (IND) application for&...
April 22, 2025 | News
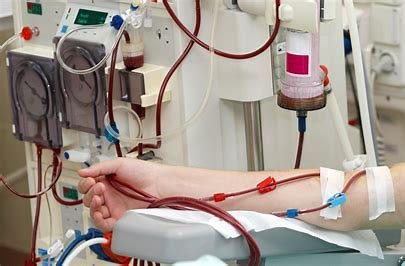
China Rises as Global Force in Blood Purification Tech at BPPC Annual Conference
Blood Purification Professional Committee (BPPC) of the China Medical Device Industry Association (CMDIA) gathered in Liuyang, Hunan, for its annual c...
April 21, 2025 | News
Akeso Secures NMPA Approval for Ebdarokimab in Moderate-to-Severe Plaque Psoriasis
Akeso, Inc. (9926.HK) ("Akeso" or the "Company") is pleased to announce that ebdarokimab, an investigational monoclonal antibody developed by the company, ...
April 21, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News