Countries China
Remegen’s Telitacicept Hits Phase III Milestone in Primary Sjögren’s Syndrome
Remegen (688331.SH/09995.HK) announced that its global first-in-class BLyS (BAFF)/APRIL dual-target fusion protein drug, Telitacicept, met the primary endp...
August 14, 2025 | News
Fosun Pharma Licenses XH-S004 to Expedition in US$645M Deal
Fosun Pharma announced that its subsidiary Fosun Pharma Industrial has signed a License Agreement with Expedition Therapeutics Inc.(Expedition) t...
August 13, 2025 | News
WuXi Biologics Achieves Fully Automated Continuous Drug Substance Manufacturing at Pilot Scale with WuXiUP™ Platform
Building on its success in developing continuous production at pilot-scale with the WuXiUP™ platform, WuXi Biologics has further enhanced the ...
August 13, 2025 | News
Mabwell Doses First U.S. Patient in Global Trial of Novel Nectin-4 ADC for Hard-to-Treat Triple-Negative Breast Cancer
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced the first patient dosing in the U.S. for a clinical stud...
August 13, 2025 | News
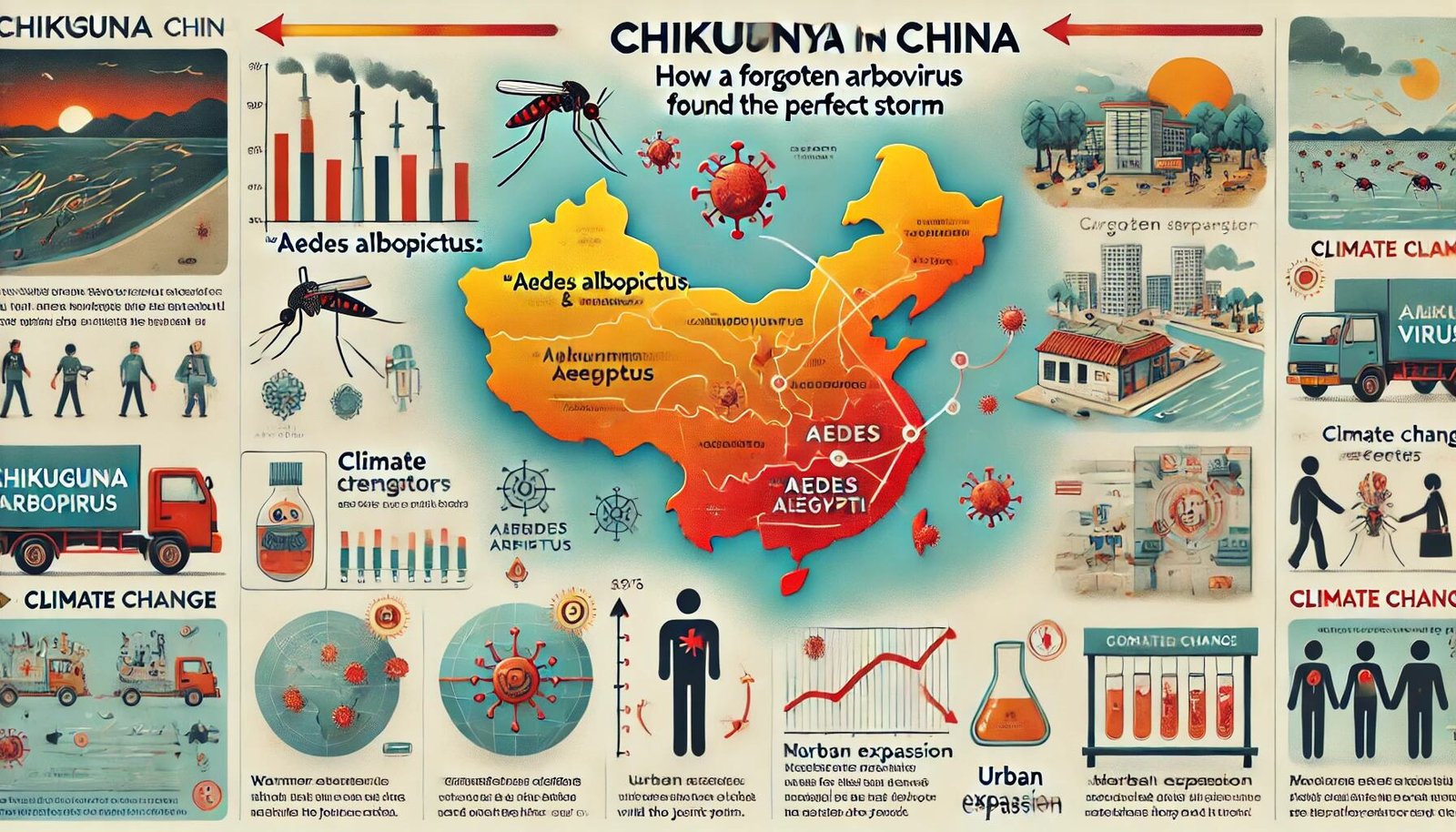
Chikungunya in China: How a “Forgotten” Arbovirus Found the Perfect Storm
Local health authorities reported thousands of cases within weeks; by early August, figures converged around the several-thousand mark, with some reports t...
August 11, 2025 | Analysis
RemeGen Secures FDA Clearance for Phase II Trials of Innovative Bispecific Antibody RC148 in Advanced Solid Tumors
RemeGen Co., announces clearance of IND application by Food and Drug Administration (FDA) for phase II clinical trials for its independently-developed bisp...
August 11, 2025 | News
GenScript Launches ‘Scripting Possibilities’—A Bold Global Brand Identity to Power the Future of Life Sciences
GenScript Biotech Corporation (HK.1548), a global leader in life sciences innovation, unveiled its new global brand platform: Scripting Possibilities...
August 11, 2025 | News
Minghui Pharmaceutical Secures $131M Pre-IPO Funding to Accelerate Global Pipeline
Minghui Pharmaceutical ("Minghui"), a late-stage clinical biopharmaceutical company, today announced the closing of a USD 131 million Pre-IPO fin...
August 08, 2025 | News
Biocair Expands APAC Footprint with New Shanghai Office to Meet Soaring Life Science Logistics Demand
Biocair, an established global leader in life science logistics, announces the further expansion of its operations in the APAC region with the opening...
August 07, 2025 | News
Fapon Biopharma Enrolls First Patient in China Phase I Trial of Novel Immunotherapy FP008 for Solid Tumors
Fapon Biopharma, a biotech in developing therapeutic antibodies and fusion proteins, is delighted to announce the completion of the first patient enrollmen...
August 06, 2025 | News
Bracco Imaging’s SonoVue® Gains NMPA Approval for HyCoSy Use in Infertility Diagnosis in China
Bracco Imaging, a global leader in diagnostic imaging,announced that its ultrasound contrast agent SonoVue® has been approved by the China's&...
August 06, 2025 | News
Innovent Biologics gets FDA nod to begin Phase 1 trials of oral GLP-1R agonist IBI3032
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality me...
August 05, 2025 | News
METiS Technologies Secures RMB 400 Million in Series D Funding to Advance AI-Powered Nanodelivery and Accelerate CGT Innovation
METiS Technologies, a global leader in AI-driven nanodelivery innovation, today announced the successful completion of its RMB 400 million Series...
August 05, 2025 | News
WuXi XDC’s New DP3 Facility Achieves GMP Release, Boosting Global Bioconjugate Manufacturing Capacity
WuXi XDC Cayman Inc. ("WuXi XDC", stock code: 2268.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO) focused on t...
August 05, 2025 | News
Most Read
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Avantor’s New CEO Ligner Aims to Unlock Global Potential and Deliver Shareholder Value
- AstraZeneca Commits $50 Billion to U.S. Expansion by 2030 in Biggest-Ever Global Investment
- Thermo Fisher, SAMRC, and South Africa’s Department of Science and Innovation Launch CATIR to Nurture Next-Gen Scientists
- Cube Biotech Appoints Former Sartorius CEO Dr. Joachim Kreuzburg to Board of Directors
- FDA’s AI Transition Marks a Turning Point in Drug Review: Industry Faces Pressure to Adapt Amid 20% Workforce Cut
- WuXi XDC Completes Mechanical Build of Singapore Bioconjugate Manufacturing Hub
News
Editor Picks