Contract Services Methods Development
WuXi XDC Delivers 62% Revenue Growth and Record Project Momentum in H1 2025
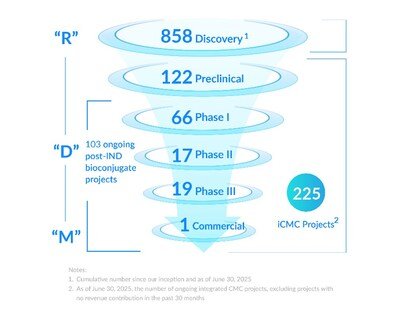
- Revenue increased by 62.2% YoY to RMB 2,701 million - Gross profit surged by 82.2% YoY to RMB 975 million, with i...
August 19, 2025 | Company results
WuXi Biologics Achieves Fully Automated Continuous Drug Substance Manufacturing at Pilot Scale with WuXiUP™ Platform
Building on its success in developing continuous production at pilot-scale with the WuXiUP™ platform, WuXi Biologics has further enhanced the ...
August 13, 2025 | News
ProBio Launches GMP AAV Manufacturing Services at New Jersey Facility to Accelerate Gene Therapy Development
ProBio, a leading contract development and manufacturing organization (CDMO) specializing in gene and cell therapy, announced the launch of its cGMP Adeno-...
August 12, 2025 | News
PolyActiva Launches U.S. Trial of Glaucoma Implant Following Australia Success
PA5108, a new chemical entity, is an investigational, biodegradable ocular micro implant, designed to deliver sustained intraocular pressure control for ...
August 08, 2025 | News
AGC Biologics Partners with Valneva to Advance Phase II Shigella Vaccine Supply from Heidelberg Site
AGC Biologics, your friendly CDMO expert, announced a development and manufacturing services agreement with specialty vaccine company Valneva SE ...
July 31, 2025 | News
I Peace and Vita Therapeutics Partner to Develop Universal Hypoimmune iPSC Lines for Cell Therapy
Leading GMP cell CDMO I Peace, Inc. specializing in induced pluripotent stem cells (iPSCs) and iPSC-derived cell therapies, announced that the co...
July 11, 2025 | News
WuXi Biologics Launches WuXiHigh™2.0 to Enable Record-Setting High-Concentration Biologic Formulations
The WuXiHigh™2.0 technology platform harnesses proprietary excipient blends and expertise to enable concentrations up to 230 mg/mL It reduces visc...
July 01, 2025 | News
Vetter Breaks Ground on New Clinical Manufacturing Site in Illinois to Expand U.S. Operations
Production site expected to be fully operational by the end of 2029 Modern site with holistic approach for state-of-the-art manufacturing operations Fu...
June 30, 2025 | News
AGC Biologics Launches Cell Therapy Manufacturing Services in Yokohama to Expand Global CDMO Footprint
AGC Biologics, your friendly CDMO expert, will commence cell therapy process development and clinical manufacturing services on July 1, 2025, at AGC Inc....
June 26, 2025 | News
Sanyou Biopharmaceuticals and TransRecoBio Form Strategic Alliance to Accelerate Innovative Formulation Development
Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. ("Sanyou Biopharmaceuticals") and TransRecoBio (Wenzhou) Biotechnology Co., Ltd. ("TransRecoBio") officially...
May 14, 2025 | News
Altaris Unites WuXi and Minaris Assets to Launch Minaris Advanced Therapies as Global Cell Therapy CDMO Powerhouse
Through strategic acquisitions made by New York–based investment firm Altaris, Minaris Regenerative Medicine and the U.S. and U.K. operations of WuXi...
May 08, 2025 | News
Genezen to Manufacture Lentiviral Vector for Optieum’s CAR-T Therapy Targeting Glioblastoma
Genezen, a leading viral vector Contract Development and Manufacturing Organization (CDMO), and Optieum Biotechnologies, Inc. (Optieum), a preclinical stag...
May 06, 2025 | News
PCI Pharma Services Acquires Ajinomoto Althea’s Altair to Build Global Powerhouse in Aseptic Filling and Advanced Drug Delivery
PCI Pharma Services ("PCI") is pleased to announce the launch of Ajinomoto Althea, the world's leading contract research and manufacturing organi...
May 05, 2025 | News
Porton Advanced Supports IND Approval of Tasly’s Dual-Targeting CAR-T Therapy for Recurrent Glioblastoma
Porton Advanced proudly announces its CDMO support for Tasly Pharmaceutical Co., Ltd.'s innovative CAR-T therapy, "P134 Cell Injection," which has received...
April 17, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News