Clinical & Diagnostic
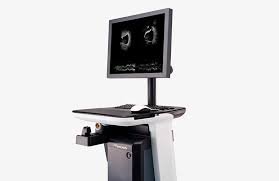
Terumo Receives FDA Clearance for OPUSWAVE Dual Sensor Imaging System Combining OFDI and IVUS Technologies
OPUSWAVE Imaging System combines OFDI and IVUS into a single catheter, providing significant potential to reduce procedural time and costs ...
October 27, 2025 | News
BioMed X and Daiichi Sankyo Launch New Research Team in Heidelberg to Advance Multispecific Biologics for Solid Tumour Immunotherapy
BioMed X, a leading innovation hub for pharma,announced the start of a new research team at its Heidelberg institute for multispecific biologics in th...
October 24, 2025 | News
Takeda and Innovent Biologics Announce Global Collaboration for Two Late-Stage Oncology Programs Valued at Over US$1.2 Billion
Takeda to Receive Rights to Two Next-Generation Late-Stage Investigational Medicines, Worldwide Outside of Greater China, and an Exclusive Option to Li...
October 23, 2025 | News
Forlong Biotechnology and Henlius Join Forces to Develop Targeted Cytokine-Based Cancer Immunotherapy
Forlong Biotechnology, a clinical-stage biotech company focusing on developing transformative cytokine therapies for patients with severe unmet needs, toda...
October 23, 2025 | News
Samsung Bioepis and Phrontline Partner to Develop Next-Generation Bispecific, Dual-Payload ADC Therapies for Solid Tumours
Samsung Bioepis and Phrontline will co-develop two investigational antibody-drug conjugate (ADC) assets directed against targets expressed in a broad r...
October 22, 2025 | News
Alphamab Oncology and CSPC’s HER2-Targeting ADC JSKN003 Receives Breakthrough Therapy Designation in China for Advanced Colorectal Cancer
Alphamab Oncology (stock code: 9966.HK) and CSPC Pharmaceutical Group Co., Ltd. ("CSPC") (Stock Code: 1093.HK) jointly announced that biparatopic HER2-targ...
October 21, 2025 | News
Sanofi’s Efluelda (Fluzone High-Dose) Cuts Influenza Hospitalisations by 31.9% in Adults Aged 65+
Compared to standard-dose influenza vaccines, Efluelda/Fluzone High-Dose demonstrated a reduction in laboratory-confirmed influenza hospitalizations by a...
October 21, 2025 | News
PeptiDream, PDRadiopharma, and Curium Launch Registrational Phase 2 Trial in Japan for 64Cu-PSMA-I&T Targeting Prostate Cancer
PeptiDream Inc., a public Kanagawa, Japan-based biopharmaceutical company (President: Patrick C. Reid, hereinafter "PeptiDream")(Tokyo: 4587), PDRadiopharm...
October 17, 2025 | News
Mabwell’s CDH17-Targeting ADC 7MW4911 Receives NMPA IND Clearance for Advanced Solid Tumours
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its self-developed CDH17-targeting ADC (R&D cod...
October 15, 2025 | News
Ascletis Selects ASC35 as Once-Monthly, Dual GLP-1/GIP Peptide Agonist Candidate for Obesity Treatment
- In head-to-head non-human primate (NHP) studies, average observed half-life of ASC35 was approximately 14 days, 6-fold longer than tirzepatide, whic...
October 14, 2025 | News
Everest Medicines Secures Dual IND Approvals for EVM14, Its First Internally Developed Therapeutic mRNA Cancer Vaccine
EVM14 becomes Everest Medicines' internally developed therapeutic mRNA cancer vaccine to receive IND approvals from both China's National Medic...
October 14, 2025 | News
Lundbeck’s Bexicaserin Receives Breakthrough Therapy Designation in China for Severe Childhood Epilepsies
Accelerated pathway underscores urgent need for innovative solutions for patients with devastating, childhood-onset epilepsies Novel 5HT2C mechanism des...
October 14, 2025 | News
Henlius’ HANSIZHUANG Meets Primary Endpoint in Phase 3 Gastric Cancer Trial
The phase 3 clinical trial of HANSIZHUANG for perioperative gastric cancer treatment met its EFS primary endpoint, supporting an early New Drug Applicati...
October 13, 2025 | News
Renalys Pharma Completes Primary Endpoint Data Collection in Phase III Sparsentan Trial for IgA Nephropathy
Renalys Pharma, Inc. (Headquarters: Chuo-ku, Tokyo; "Renalys") announced that it has completed data collection for the primary endpoint in the Phase I...
October 13, 2025 | News
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News