China
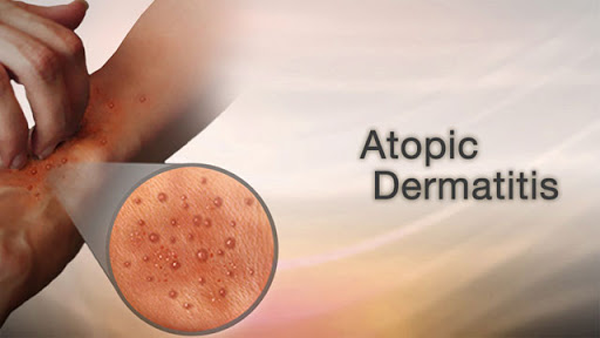
Minghui Pharma's MH004 Cream Succeeds in Phase 2 Trial for Atopic Dermatitis, Global Phase 3 MRCT Cleared by FDA
Study met both primary and all key secondary endpoints Mean percentage change from baseline in Eczema Area and Severity Index (EASI) score was -78.7% in...
April 14, 2023 | News
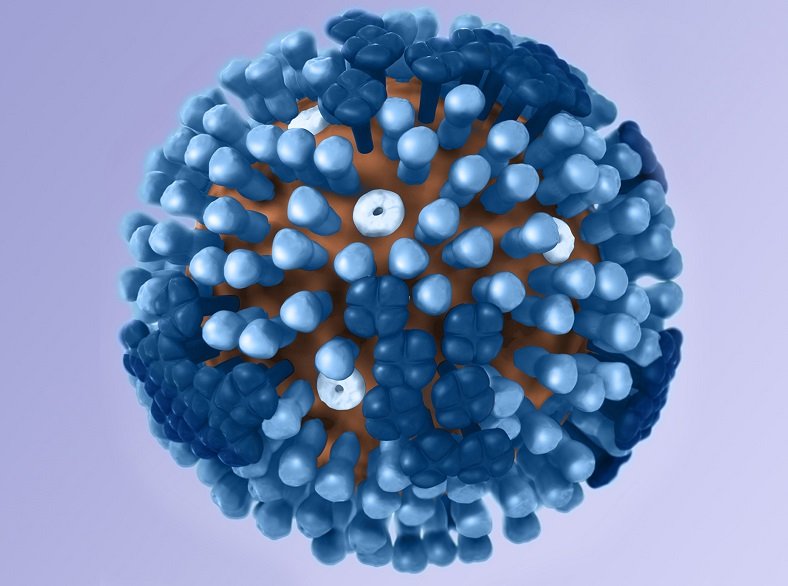
Zenshine's ZX-7101A meets primary endpoint in Phase 2 flu treatment study
Interim analysis of the phase 2 cohort of ZX-7101A phase 2/3 adaptive design pivotal study (registration number: CTR20221729), a multicenter, randomized, d...
April 14, 2023 | News
CPHI Japan: the rise of Chinese innovation the big macro story for APAC in 2023
“Historically, we have seen a dual strategy of focusing on China and the US for emerging innovators in China. However, a series of recent setbacks wi...
April 13, 2023 | News
Biosyngen received China NMPA IND approval for its T-cell redirection therapy targeting EBV-positive Lymphoma
Currently, commercially available cell therapies, such as CD19 CAR-T, are designed for the treatment of B cell lymphoma or acute lymphoblastic leukemia. Tr...
April 12, 2023 | News
Boan Biotech's Biologics License Application for Boyounuo® (Bevacizumab Injection) Accepted in Brazil
Envisioned to be "a leading global biopharmaceutical company", Boan Biotech develops biologics for both Chinese and international markets, including E...
April 12, 2023 | News
JW Therapeutics' Relma-cel approved for systemic lupus erythematosus trial.
SLE is a complex autoimmune disease with diverse clinical manifestations involving many organs and systems. It is estimated that China has 1 mill...
April 11, 2023 | News
Asieris subsidiary obtains "Drug Distribution License" for commercialization strategy.
Hainan Asieris' acquisition of the Drug Distribution Licence is a critical step towards improvement in the quality of drug circulation services, standardiz...
April 11, 2023 | News
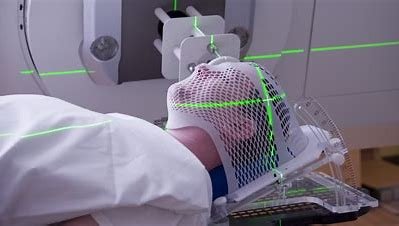
Chinese NMPA Approves Study of Telix Brain Cancer Therapy Candidate
The investigational new drug (IND) application was submitted by Telix's partner in the Greater China region, Grand Pharmaceutical Group Limited (...
April 11, 2023 | News
Evopoint, MSD Partner for Clinical Trial of XNW5004 with KEYTRUDA in Solid Tumors.
XNW5004 is a rationally designed, highly selective and potent EZH2 inhibitor with potentially best-in-class efficacy and safety profile demonstrated in a P...
April 10, 2023 | News
China's First Nectin-4 Targeted ADC 9MW2821 Clinical Progress Released
Developed with site specific conjugate technology, 9MW2821 is China's first, global second Nectin-4 targeted ADC approved for clinical study. The...
April 08, 2023 | News
First patient dosed in China Phase III trial for COPD, announces Nuance Pharma
Ensifentrine is a first-in-class, selective, dual inhibitor of the enzymes phosphodiesterase 3 and 4 ("PDE3" and "PDE4") combining bronchodilator and non-s...
April 07, 2023 | News
Singapore HSA Accepts Everest Medicines' Nefecon Application for IgA Nephropathy Treatment
Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commerc...
April 06, 2023 | News
WuXi STA Adds New Peptide Manufacturing Capacity and Large-Scale Continuous Purification System
WuXi STA, a global Contract Research, Development, and Manufacturing Organization (CRDMO), announces that it has added two 2,000 L reactors and a lar...
April 06, 2023 | News
Biosion Initiates Phase II Clinical Trial of BSI-045B in Atopic Dermatitis
"BSI-045B is a next generation anti-TSLP mAb with high affinity and bioactivity that has the potential to offer greater therapeutic potential for patients ...
April 05, 2023 | News
Most Read
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- How Health Systems Are Reshaping Drug Adoption, Partner Models, and Market Access in 2026
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News