Helping to maintain the pace of innovation with aptamers
12 December 2022 | Monday | Analysis

Part of this change in approach has led to an increased interest in aptamer technology in place of traditional antibodies. Like antibodies, aptamers can be used across a wide variety of affinity applications and are being explored as discovery reagents, diagnostic tools, and as therapeutics.
Oligonucleotide aptamers are chemically synthesized analogs of antibodies. Generated from diverse libraries of DNA or RNA, they fold to form specific sequence-dependent structures that allow specific interaction with a target of interest. Aptamers offer several advantages over traditional antibodies (table 1), including faster discovery, improved stability, and chemical synthesis for improved batch compatibility.
|
Aptamer |
Antibodies |
||
|
Stability |
|
|
|
|
Preparation |
|
|
|
|
Target potential |
|
|
|
|
Size |
|
|
|
|
Modifiability |
|
|
|
|
Affinity |
|
|
|
|
Specificity |
|
|
|
|
Tissue uptake/Renal filtration |
|
|
|
Comparative features of oligonucleotide aptamers and antibodies
Several important paradigms have been demonstrated in the field of therapeutic research. Firstly, like monoclonal antibodies, aptamers can act as inhibitors of protein function, interfering with the aberrant function of a cellular processes, and can therefore be applied directly as therapeutic agents.1,2 Secondly, aptamers can be developed to target cell-specific surface markers, which are then internalized through mechanisms such as receptor-mediated endocytosis. This makes aptamers valuable tools for the targeted delivery of small nucleic acid-based therapies, such as siRNA and ASOs, as well as conventional small-molecule drugs.3 Finally, aptamers are readily coupled with liposomes and other carriers to form smart delivery systems for the targeted delivery of small molecules, peptides, oligonucleotides, and the CRISPR/Cas9 system for gene editing.
Accelerating the journey to the clinic
Recent research has shown that compared to traditional antibody discovery and development, using aptamer-based approaches can reduce the timeline from discovery to the clinic by as much as 70%.4 This shorter discovery and development timeline enables developers to expedite critical medicines into the clinic while gaining a competitive advantage by entering clinical trials earlier than other products.
Securing supply chains
Beyond the faster development, another critical factor highlighted by the pandemic was the fragility of many supply chains. With the increased pressure placed on cold chain supply (essential to maintain the stability of antibodies), the supply of many affinity reagents was interrupted. Aptamers offer improved stability and can be shipped in a readily reconstituted, lyophilized state at room temperature. This negates the need for end-to-end cold chain supply and allows simpler global logistics.
Tuning therapeutic half
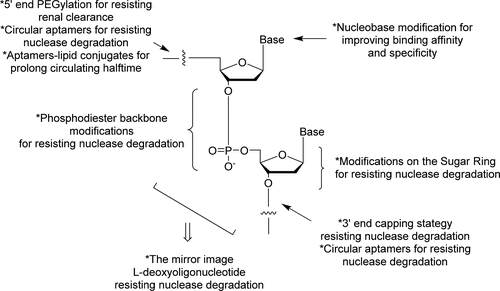 Methods of aptamer modulation to increase in vivo half-life
Methods of aptamer modulation to increase in vivo half-life
A concern in the early stages of therapeutic aptamer development was their short half-life in vivo. This was due to their susceptibility to degradation by nuclease activity and small molecular weight, which allowed rapid glomerular filtration. The field moved quickly to address these issues through various strategies, including incorporating modifications on the sugar ring of the nucleic acid building blocks, e.g., 2’fluoro, 2’O-Methyl groups. These modifications increase the resistance to endogenous nucleases for longer therapeutic half-lives. With these stability increases, it is now possible to tune the half-life of aptamer therapeutics according to the desired pharmacokinetic profile. Unmodified aptamers are being applied to treat acute conditions for rapid hit-and-run strategies, and modified aptamers are applied for longer half-lives as required for relevant indications.
To overcome the renal filtration issue, therapeutic aptamers can be conjugated to large inert groups like cholesterol or polyethylene glycol (PEG) or multimerized to generate bi-specific or multi-specific aptamers. While the conjugation of PEG groups is a convenient solution, there are multiple reports of pegylation leading to immunogenic reactions.5 Multimerisation of aptamers overcomes potential immunogenicity stemming from additional chemical groups and increases the molecular weight beyond the 30-50 kDa cut-off for renal filtration.6 Multimerization of a single aptamer can increase binding through avidity effects, whereas multimerization of different aptamers can allow targeted delivery to the site-of-action through the development of bi-specific aptamers, with one aptamer as the therapeutic portion and a second as the targeting vehicle to direct the therapeutic to the desired cell type.
Hitting new targets and challenging tissues
Since the approval of the first antibody therapeutic in 1986, biologic developers have pursued an ever-broadening target range. As of 30 June 2022, antibody therapeutics for over 90 different protein targets have been approved for treatment globally.7 Considering there are around 20,000 human protein-coding genes, this target range could surely be expanded upon to offer new treatments for a broader range of conditions.
Aptamers can target a wide spectrum of molecules, from small molecules to cells, including targets with weak or no immunogenicity that cannot be recognized by antibodies, and toxins that may kill an animal before an immune response and associated antibodies can be raised. This presents a greater target range for aptamers to explore for therapeutic potential. The ability to routinely develop aptamers to small molecules opens new therapeutic avenues unavailable to protein-based reagents, such as inducible gene therapies.8 Further, there is the potential to carry out aptamer discovery against diseased vs. healthy cells, without targeting specific biomarkers, to allow the identification of new disease biomarkers and the corresponding affinity ligand within the same process.

Aptamer discovery can be performed to specific protein or cell biomarkers or in a hypothesis-free manner to identify new disease biomarkers.3
Combining the best features of therapeutic size
Aptamers can act as small molecules with superior flexibility and infiltrate target sites that may not be accessible to larger antibody molecules. Meanwhile, aptamers can also be applied in targeted therapies, offering selectivity that cannot be achieved with small-molecule drugs. These superior properties give aptamers a niche in the therapeutic field between small-molecule drugs and high-molecular-mass antibodies. Combining this characteristic with the possibility of controlling the design of each aptamer therapeutic through in vitro discovery and rapid development is helping to maintain the innovation and pace of discovery across the life sciences.
References
- Hernández-Jiménex, M. et al. Mol Ther Nucleic Acids 2022, 28:124-135
- Becker, N.P. et al. Clin Drug Invest 2020, 40:433–447
- Aptamer Group. Optimer®-drug conjugates Available at: https://aptamergroup.com/wp-content/uploads/2022/08/Optimer-drug-conjugates-AG.pdf
- Aptamer Group. Optimer®: Accelerating your journey to the clinic. Available at: https://mailchi.mp/aptamergroup.com/l75ey5r3z9
- Lincoff, A.M. et al. Lancet 2016, 387:349– 356
- Moreno, A. et al. Cell Chemical Biology 2019, 26(5):634-644
- Lyu, X. et al. Antib Ther 2022, 5(4):233–257
- Aptamer Group. Aptamer Group signs deal with Flip Gene Therapeutics to support the development of inducible gene therapies. Available at: https://aptamergroup.com/aptamer-group-signs-deal-with-flip-gene-therapeutics-to-support-the-development-of-inducible-gene-therapies/
Most Read
- How Does GLP-1 Work?
- Innovations In Magnetic Resonance Imaging Introduced By United Imaging
- Management of Relapsed/Refractory Multiple Myeloma
- 2025 Drug Approvals, Decoded: What Every Biopharma Leader Needs to Know
- BioPharma Manufacturing Resilience: Lessons From Capacity Expansion and Supply Chain Resets from 2025
- APAC Biopharma Review 2025: Innovation, Investment, and Influence on the Global Stage
- Top 25 Biotech Innovations Redefining Health And Planet In 2025
- The New AI Gold Rush: Western Pharma’s Billion-Dollar Bet on Chinese Biotech
- Single-Use Systems Are Rewiring Biopharma Manufacturing
- The State of Biotech and Life Science Jobs in Asia Pacific – 2025
- Asia-Pacific Leads the Charge: Latest Global BioSupplier Technologies of 2025
- Invisible Threats, Visible Risks: How the Nitrosamine Crisis Reshaped Asia’s Pharmaceutical Quality Landscape
Bio Jobs
- Sanofi Turns The Page As Belén Garijo Steps In And Paul Hudson Steps Out
- Global Survey Reveals Nearly 40% of Employees Facing Fertility Challenges Consider Leaving Their Jobs
- BioMed X and AbbVie Begin Global Search for Bold Neuroscience Talent To Decode the Biology of Anhedonia
- Thermo Fisher Expands Bengaluru R&D Centre to Advance Antibody Innovation and Strengthen India’s Life Sciences Ecosystem
- Accord Plasma (Intas Group) Acquires Prothya Biosolutions to Expand Global Plasma Capabilities
- ACG Announces $200 Million Investment to Establish First U.S. Capsule Manufacturing Facility in Atlanta
- AstraZeneca Invests $4.5 Billion to Build Advanced Manufacturing Facility in Virginia, Expanding U.S. Medicine Production
News











